
Bidirectional regulation between tumor cell-intrinsic PD-L1 and TGF-β1 in epithelial-to-mesenchymal transition in melanoma
Introduction
Melanoma is a potentially growing public health problem across the world with a continuing increase in incidence and mortality rates. However, the current promising immunotherapy strategy anti-programmed death receptor-1 (PD-1)/PD ligand-1 (PD-L1) antibodies, which interrupts the interaction between immune checkpoints expressed on tumor and immune cells, showed response rates in the range of only 30–40% (1-3). Moreover, emerging evidence shows that tumor cell-intrinsic PD-L1 promotes tumor cell proliferation, facilitates self-renewal of cancer stem cells in an immune-independent way in melanoma, and PD-L1 signaling also played a crucial role in maintenance of the epithelial-to-mesenchymal transition (EMT) in various cancers (4-7), which suggested that tumor cell-intrinsic PD-L1 signaling needed to be further explored.
The previous researches documented that increased transforming growth factor-β (TGF-β) signaling in the tumor microenvironment (TME) was correlated with poor overall survival and resistance to inhibition of PD-1/PD-L1 (8-10). With regard to the multi-functions of TGF-β, high-quality review articles have been published (11,12). For short, TGF-β signaling pathway promotes tumor progression, favors EMT, suppresses the sensitivity to anticancer drugs, represses the anti-tumor functions of various immune cells and limits the efficacy of immunotherapeutic approaches. Theoretically, three highly homologous TGF-β isoforms exist in mammals: TGF-β1, TGF-β2 and TGF-β3. Among them, TGF-β1 is the predominant form of TGF-β in the TME. TGF-β1 blockade can act as a monotherapy through a tumor-intrinsic effect blocking the EMT-like transition (13). Nevertheless, clinical studies with anti-TGF-β agents have led to limited success (13-15). To address this issue, bifunctional anti-PD-L1/TGF-β trap fusion protein (bintrafusp alfa, also referred as M7824) has been innovatively developed and has shown a promising antitumor efficacy in patients with heavily pretreated advanced solid tumors with a manageable safety profile similar to anti-PD-1/PD-L1 monotherapies (13,16,17).
Although there still lacked clinical trial data of M7824 in patients with melanoma, TGF-β1 gene signatures were indeed found to be associated with resistance to PD-1/PD-L1 blockade in melanoma patients (13). However, more explicit interaction between PD-L1 and TGF-β1 in melanoma is still unknown, which is vital for the future research of bintrafusp alfa in the utilization on melanoma patients. In the present study, we explored the relationship between tumor cell-intrinsic PD-L1 and TGF-β1 in melanoma and found that (I) there existed a bidirectional regulation between the melanoma surface expression of PD-L1 and the secretion of melanoma-derived TGF-β1, (II) PD-L1 promoted the TGF-β-induced EMT and progression both in vitro and in an animal model. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-292/rc).
Methods
Cell lines and cell culture
The mouse melanoma cell line B16-F0 was obtained from Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (catalog ZQ0506, Shanghai, China). B16-F10 melanoma cell line was donated by the Cancer Center of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. Cells were cultured in RPMI-1640 (Gibco, USA) containing 10% fetal bovine serum (FBS) (NQBB, China), 100 U/mL penicillin and 100 µg/mL streptomycin (Biosharp, China) at 37 ℃ in 95% humidity and 5% CO2. Recombinant Mouse TGF-β1 (catalog 7666-MB, R&D Systems, USA, 5 ng/mL), TGF-β1 inhibitor SB431542 (catalog 1614, Tocris Bioscience, USA, 10 Um) and purified anti-mouse CD274 (catalog 124302, BioLegend, USA, 5 µg/mL) was added in the corresponding groups.
Flow cytometry
The PE Rat Anti-mouse CD274 antibody was purchased from BD Pharmingen (catalog 558091, BD Bioscience, USA) and used for the flow cytometry analysis. Cells were treated with 48 hours of Recombinant Mouse TGF-β1 (catalog 7666-MB, R&D Systems, USA, 5 ng/mL), TGF-β1 inhibitor SB431542 (catalog 1614, Tocris Bioscience, 10 Um), or dimethylsulfoxide (DMSO) (10 Um) as control respectively. Then cells were centrifuged after 1–2 minutes of 0.25% trypsin digestion (catalog G4002, Servicebio, China), resuspended in ×1 phosphate buffered saline (PBS), incubated with CD274 antibody at 4 ℃ for 30 minutes. The cells were then washed and resuspended in PBS. All flow cytometry analysis were performed on BD LSRFortessaTM X-20, and the data were analyzed using FlowJo software.
Real-time quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted using TRIzol (TaKaRa, China) according to the standard procedures. The RNA was used as template for reverse transcription to synthesize cDNA using HiScript III RT SuperMix reagent (Vazyme, China). The RT-qPCR was performed according to the following conditions using the AceQ qPCR SYBR Green Master Mix (Vazyme, China): initial denaturation at 95 ℃ for 5 minutes, followed by 40 cycles at 95 ℃ for 10 seconds and 60 ℃ for 30 seconds, finally at 95 ℃ for 15 seconds, 60 ℃ for 60 seconds and 95 ℃ for 15 seconds. The PCR reaction was monitored on ABI StepOneplusTM Real Time PCR System (ThermoFisher, USA). The GAPDH gene was used as internal reference to normalize the results and mRNA expression was calculated through relative quantification (2−ΔΔCT). The primer sequences were designed using Primer-BLAST. The primer sequences were as follows: GAPDH, forward 5'-GAGAAGGCTGGGGCTCATTT, reverse 5'-AGTGATGGCATGGACTGTGG. PD-L1, forward 5'-CAGCAACTTCAGGGGGAGAG, reverse 5'-TTTGCGGTATGGGGCATTGA. TGF-β1, forward 5'-AGCTGCGCTTGCAGAGATTA, reverse 5'-AGCCCTGTATTCCGTCTCCT. E-Cadherin (CDH1), forward 5'-AACCCAAGCACGTATCAGGG, reverse 5'-ACTGCTGGTCAGGATCGTTG. Fibronectin 1 (FN1), forward 5'-ATGAGAAGCCTGGATCCCCT, reverse 5'-CAGTTGGGGAAGCTCATCTGT.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of TGF-β1 in cell culture supernatant and tissue homogenates were detected by double antibody sandwich ELISA method with the Mouse/Rat/Porcine/Canine TGF-β1 Valukine ELISA Kit (catalog VAL611, Bio-Techne, China). The anti-mouse TGF-β1 monoclonal antibody has been precoated and fixed on the microplate. The unbound substances were washed. The anti-mouse TGF-β1 polyantibody labeled with horseradish peroxidase was added, and the unbound antibody-enzyme was washed. The substrate solution (chromogenic agent) was added to the wells and the depth of the solution color was positively correlated with the concentration of TGF-β1 in the sample. Finally, the terminating solution was added and the absorbance was determined by enzyme labeling instrument.
Cell transduction assays
The B16-F0 and B16-F10 cells were infected with shRNA lentiviruses encoding mouse PD-L1 (PD-L1-shRNA) or meaningless negative control sequence (NC-shRNA) as control coupled with green fluorescent protein (GFP), respectively; 2×104 cells were seeded into each well of a 12-well plate on the day before lentiviruses transduction. The ratio of virus to cell number, namely the multiplicity of infection (MOI), was 40. After 24 hours of infection, the culture medium was removed and replaced with fresh medium for 36 hours. The cells were screened with RPMI-1640 medium containing 3 µg/mL puromycin. The GFP+ cells were observed under a fluorescence microscope and the efficiency of lentivirus infection was calculated.
Cell proliferation assay
Cell proliferation was measured by cell counting kit-8 (CCK-8, Biosharp, China); 2×103 cells were inoculated in 96-well plate at a 100 µL volume of each well. After 48 hours, 10 µL CCK-8 solution was added to each well and incubated for 2 hours. The absorbance was assessed at 450 nm by enzyme labeling instrument.
In vitro scratch assay
For scratch assays, 5×105 cells were suspended in RPMI-1640 containing 10% heat-inactivated FBS and plated in 6-well plates. After 24 hours, a scratch wound was made using sterile pipet tips across each well. Cultures were gently washed with PBS to remove loose cells. The cells were then maintained in RPMI-1640 containing 0.1% FBS with or without recombinant mouse TGF-β1 (catalog 7666-MB, R&D Systems, USA) at the concentration of 5 ng/mL for 24 hours. Immediately after the scratch, images of the scraped area were captured using phase contrast microscopy. The scratch width at six different points per image were measured.
Animals and tumor model
PD-L1-shRNA and NC-shRNA melanoma cells (B16-F0 and B16-F10) in logarithmic growth stage were collected and resuspended at a final concentration of 2.5×106 cells/mL. C57BL/6 mice (7-week-old, male) were sourced from Beijing Vital River Laboratory Animal Technology Co., Ltd. and housed in specific pathogen free (SPF) environment in laboratory animal center, Huazhong University of Science and Technology. All animals were randomly divided into two groups (B16-F0 and B16-F10 group), 6 in each group. And the mice in each group were randomly divided to be inoculated PD-L1-shRNA melanoma cells and NC-shRNA melanoma cells as control subcutaneously with 200 µL suspension containing 5×105 cells, respectively. The tumor size was measured every other day. Tumor volume was calculated according to the following formula: tumor volume =0.52× tumor length diameter × (tumor short diameter)2. Mice were sacrificed under deep anesthesia 23 days after inoculation. Then the growth curve was plotted according to tumor volume. Experiments were performed under a project license (No. 2837) granted by Institutional Animal Care and Use Committee Board of Huazhong University of Science and Technology, in compliance with institutional guidelines for the care and use of animals.
Immunohistochemistry
Mice were sacrificed on indicated days. Tumor tissues were completely dissected, fixed in 4% buffered paraformaldehyde, embedded in paraffin, and were subsequently cut into 5-µm-thick sections. The sections were dewaxed and then placed in citric acid buffer (PH 6.0) for microwave antigen retrieval. Endogenous peroxidase of sections was then blocked with 3% hydrogen peroxide solution. Sections were further blocked with 3% bovine serum albumin blocking solution (Sigma) and immunostained with rabbit anti-mouse PD-L1 (ab233482), rabbit anti-mouse α-smooth muscle actin (SMA) (ab5694), rabbit anti-FN1 (ab2413), rabbit anti-CDH1 (ab76319) and rabbit anti-Ki-67 (ab15580) purchased from Abcam, UK at 4 ℃ overnight. The sections were then washed well with phosphate buffered saline with Tween-20 (PBST) and incubated with goat anti-rabbit IgG secondary antibody (ab150077, abcam) at room temperature for 1 hours. After being washed with PBST, staining was developed with DiAminoBenzidine. Slides were counterstained with hematoxylin, dehydrated, and finally mounted. These sections were observed under a microscope and were measured by using Image-pro Plus 6.0 software.
Statistical analyses
Statistical analyses were performed using GraphPad Prism v8.0.1. Unless otherwise stated, the data were shown as the mean ± standard deviation (SD). Two-sided unpaired t-test or two-way analysis of variance (ANOVA) tests were used to evaluated the significant differences. The P value of less than 0.05 was considered to be statistically significant.
Results
TGF-β1 promoted PD-L1 expression in melanoma cells
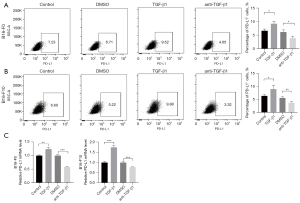
To explore the effect of TGF-β1 on the expression of PD-L1 in melanoma cell lines, B16-F0 and B16-F10 cells were both treated with TGF-β1 and TGF-β1 inhibitor respectively for 48 hours. The protein expression of PD-L1 were identified by flow cytometry. We found that PD-L1 protein expression level in B16-F0 cells was induced by TGF-β1 over 1.40-fold higher (P=0.0122) and suppressed by TGF-β1 inhibitor over 1.57-fold lower (P=0.0198) compared to those from control group (Figure 1A). Consistently, the protein expression of PD-L1 was increased by TGF-β1 over 1.41-fold higher (P=0.0488) and decreased by TGF-β1 inhibitor over 1.51-fold lower (P=0.0085) compared to those from control group in B16-F10 cells (Figure 1B). We also tested the effect of TGF-β1 on PD-L1 mRNA expression by RT-qPCR. The results exhibited that TGF-β1 increased the mRNA expression of PD-L1 in both melanoma cell lines, which was confirmed in TGF-β1 inhibitor-treated cells (Figure 1C).
PD-L1 induced the secretion of TGF-β1 in melanoma cells
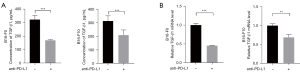
In order to determine whether a bidirectional regulation existed between PD-L1 and TGF-β1 in melanoma cells, we explored the effect of PD-L1 on the expression and secretion of TGF-β1. Cell-surface PD-L1 of B16-F0 and B16-F10 cells was blocked with purified anti-mouse CD274 (anti-PD-L1, 5 µg/mL) for 48 hours. The cultured supernatant was then collected and the total RNA of B16-F0 and B16-F10 cells were extracted. The concentration of TGF-β1 in the supernatant was detected by ELISA kit and the mRNA level of intracellular TGF-β1 was measured by RT-qPCR. The results showed that anti-PD-L1 could significantly inhibit the secretion of TGF-β1 was significantly suppressed to 1.90-fold in B16-F0 cells (P<0.0001, Figure 2A) and 1.49-fold in B16-F10 cells (P<0.0001, Figure 2A) in anti-PD-L1 group compared with those from control group. Importantly, anti-PD-L1 downregulated intracellular TGF-β1 mRNA levels to 2.20-fold in B16-F0 cells (P<0.0001, Figure 2B), and to 1.45-fold in B16-F10 cells (P=0.0011, Figure 2B), respectively. Together with above results, it was indicated that cell intrinsic PD-L1 could induced the expression and secretion of TGF-β1 in melanoma cells.
PD-L1 favored TGF-β1-induced EMT, proliferation and migration in vitro
We further analyzed the relationship between TGF-β1 and PD-L1 in mouse melanoma model. Immunohistochemical staining revealed that PD-L1 protein is expressed in both B16-F0 and B16-F10 tumor tissues. Importantly, PD-L1 protein expression were co-localized with α-SMA protein expression (Figure 3A), which suggested that PD-L1 expression was associated with EMT.
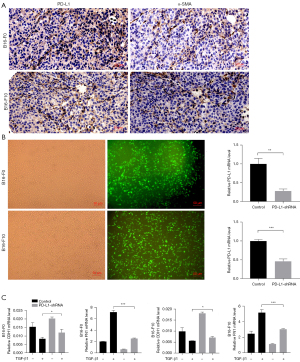
Next, we investigated whether PD-L1 was involved in TGF-β1-mediated EMT. We used shRNA lentivirus to knockdown PD-L1 in melanoma cell lines (Figure 3B) and treated with or without TGF-β1 for 48 hours. The EMT markers, CDH1 and FN1 were observed. We detected the mRNA levels of CDH1 and FN1 using RT-qPCR. The data showed that the mRNA level of FN1 was decreased in both PD-L1-shRNA B16-F0 cells and PD-L1-shRNA B16-F10 cells compared to controls (64.6%, P<0.0001 and 41.4%, P=0.0007, respectively, Figure 3C). We also found that PD-L1-shRNA B16-F0 cells and PD-L1-shRNA B16-F10 cells expressed higher levels of CDH1 mRNA than that of controls (43.7%, P=0.0434 and 26.1%, P=0.0227, respectively, Figure 3C).
Furthermore, we investigated whether PD-L1 was needed for TGF-β1-induced cell proliferation and migration. The results demonstrated that cell proliferation was inhibited by 21.3% (P<0.0001) in PD-L1-shRNA B16-F0 cells and 29.8% (P=0.0002) in PD-L1-shRNA B16-F10 cells (Figure 4A). Scratch assay in vitro is one of the most widely used methods for studying cell migration. In these experiments, PD-L1-shRNA B16-F0 cells treated with TGF-β1 showed slower migration ability than B16-F0 cells treated with TGF-β1 (17.66% vs. 67.13% wound closure, P<0.0001) at 24 hours time points (Figure 4B,4C). The similar results were observed in PD-L1-shRNA B16-F10 cells and B16-F10 cells which both treated with TGF-β1 (17.90% vs. 56.64% wound closure, P=0.0002). These results demonstrated that PD-L1 regulated TGF-β1-mediated EMT, cell proliferation and migration.

PD-L1 regulated TGF-β1-induced EMT and promoted melanoma progress in vivo
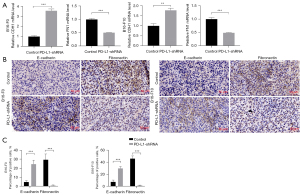
Based on the above findings, we further confirmed the effect of PD-L1 on TGF-β1-mediated protumorigenic activity in the mouse melanoma model. In PD-L1-shRNA B16-F0 melanoma model, tumor growth was significantly suppressed by 5.52-fold (P<0.001) (Figure 5A,5B). As shown in Figure 5C,5D, the growth rates of the PD-L1-shRNA B16-F10 tumors were substantially lower than those of the control tumors (2.70-fold, P<0.001). After 20–23 days, the mice were sacrificed. The tumors were weighed and homogenated. Ki-67 has been identified as a well-known protein marker to evaluate the proliferative activity of cancer cells. The data showed that shRNA-mediated PD-L1 knockdown significantly inhibited the Ki-67 expression in two melanoma models (Figure 5E,5F). The TGF-β1 concentration in supernatant was detected by ELISA assay. The data demonstrated that PD-L1-shRNA tumors secreted lower TGF-β1 levels than those in the control tumors in both B16-F0 model (2.00-fold, P<0.001) and B16-F10 model (1.61-fold, P=0.0005) (Figure 5G).
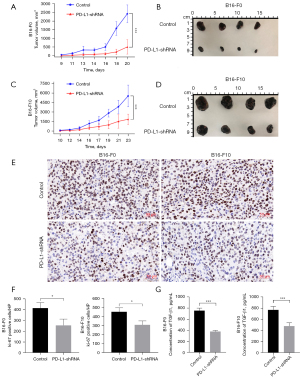
We next assessed whether shRNA-mediated PD-L1 knockdown would suppress the process of EMT in vivo. Our data indicated that the mRNA level of FN1 was markedly decreased in both B16-F0 model (1.97-fold, P<0.001) and B16-F10 model (2.04-fold, P=0.0001) compared to controls (Figure 6A). We also found that the CDH1 mRNA level was significantly increased in two melanoma model than that of controls (B16-F0 model, 3.56-fold, P=0.0001; B16-F10 model, 1.77-fold, P=0.001) (Figure 6A). The protein levels of CDH1 and FN1 were detected by immunohistochemical staining in the removed tumor tissues. As can be seen in Figure 6B,6C, CDH1 protein and FN1 protein changes were similar to that observed in mRNA levels. Taken together, these results further confirmed that tumor cell-intrinsic PD-L1 is the potent inducer of TGF-β1-mediated EMT and tumor progression.
Discussion
PD-1/PD-L1 inhibition has emerged as an indispensable choice in melanoma patients, however only less than half melanoma patients respond well to anti-PD-L1/PD-1 therapy. The reasons of primary and secondary drug resistance to PD-L1 still remained to investigate (18). In addition to immune-dependent role, understanding the regulation of expression and roles of tumor-intrinsic PD-L1 are important for further improving the response to immunotherapy. In the context of malignancy, EMT confers on cancer cells increased tumor-initiating and metastatic potential and a greater resistance to therapeutic regimens. Additionally, activation of an EMT program in carcinoma cells also leads to a tumor-initiating state (19). It has been reviewed that the bidirectional regulation between the EMT process and PD-L1 signaling led to tumor immune escape (20). Tumor cell-intrinsic PD-L1 promoted tumor cell proliferation and immune-independent growth (21,22). In the present study, we confirmed the cross-talk between cell-intrinsic PD-L1 and TGF-β1 in induction of EMT and tumor progression in melanoma, which could provide more information for dual target of PD-L1 and TGF-β in melanoma.
PD-L1 expression is regulated in tumors in various ways, in addition to being induced by interferon-γ (IFN-γ) secreted by infiltrating lymphocytes as a mechanism of immune escape (10). It has been described that PD-L1 is regulated at the transcriptional and RNA stability levels by MYC and oncogenic RAS, respectively (5,6), as well as at the protein level by the CSN5, CMTM4 and CMTM6 proteins (23,24). It has also been reported that eukaryotic translation initiation factor 4F (eIF4F), which binds to the 5’ cap of mRNAs, regulated the surface expression of IFN-γ-induced PD-L1 on melanoma cells by regulating translation of the mRNA encoding the STAT1 transcription factor (25). Recent studies have demonstrated that the EMT process induced upregulated expression of PD-L1 (7,16,20). What’s more, several other researches revealed that PD-L1 signaling played a crucial role in maintenance of the EMT status in breast cancer, renal cell carcinoma, esophageal cancer, hepatocellular carcinoma and glioblastoma (5,7,26-28). These data indicated that a bidirectional regulation existed between EMT status and PD-L1 signaling. TGF-β has been generally considered as a key inducer of EMT. Our results demonstrated that TGF-β1 induced melanoma cell-intrinsic PD-L1 expression. Furthermore, we demonstrated PD-L1 increased the synthesis and secretion of TGF-β1. These data indicated that bidirectional regulation between tumor cell-intrinsic PD-L1 and TGF-β1 in melanoma existed.
Three TGF-β isoforms are essential regulators of cell differentiation, phenotypes and functions, and are involved in the pathogenesis of many diseases by different mechanisms (11). In melanoma, all three TGF-β isoforms were significantly increased and their expression was correlated with a poor survival of patients (29). These observations highlighted TGF-β can be explored as a drug target for cancer therapy. However, TGF-β blockade can cause significant toxicity, which has led to termination of several clinical trials testing TGF-β/TGF-βRII inhibitors (13,30). Theoretically, bifunctional molecules targeting two pathways simultaneously may display their superiority compared with two single agents used in combination treatment. Anti-PD-L1/TGF-βRII (M7824) is an innovative first-in-class bifunctional fusion protein composed of a monoclonal antibody against PD-L1 fused to TGF-βRII. Clinical evaluation of M7824 indicated the adverse event profile included anti-PD-L1 and anti-TGF-β-related respective adverse events (17,31-33) but with a narrow therapeutic window, which warranted further investigation. Another study has reported that combined inhibition of PD-L1 and TGF-β1-induced EMT resensitized hepatocellular carcinoma to sorafenib treatment (27). These data suggested that TGF-β1 may serve as a potential factor for prognostic evaluation of melanoma, which may benefit from designing targeting TGF-β1 signaling along with PD-L1. Our previous findings demonstrated that PD-L1 promoted self-renewal and tumorigenicity of malignant melanoma initiating cells (34). The present results showed PD-L1 promoted melanoma progression in vitro and in vivo by TGF-β1 inducing EMT, which was highly concordant with work of others showing similar results in hepatocellular carcinoma and lung cancer (18,27). Furthermore, based on the above results we wonder whether the EMT makers in melanoma such as CDH1, FN1, MITF, ZEB1, AXL, SNAIL could serve as the predictors for anti-PD-L1 therapy sensitivity, which needs further investigation. The major weakness of our study is not involved in the molecular mechanism. We are aware that these findings should be further investigated in the molecular mechanism involved in bidirectional regulation between cell-intrinsic PD-L1 and TGF-β1. In addition, our study is limited by the absence of exploring the relationship between the other two TGF-β isoforms and PD-L1. Nevertheless, the present study showed that a bidirectional crosstalk between cell-intrinsic PD-L1 and TGF-β1 in melanoma. Furthermore, we demonstrated that PD-L1 induced TGF-β1-mediated EMT and tumor progression. Considering that the role and contribution of TGF-β isoforms are different in cancers, TGF-β1 may serve as a potential factor for prognostic evaluation of melanoma and help in designing promising combinations which include targeting TGF-β1 signaling along with PD-L1 or other immune checkpoint blockade, chemotherapy or radiotherapy.
Acknowledgments
Funding: This work was supported by a grant from
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-292/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-292/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-292/coif). FZ reports that this work was supported by a grant from the National Natural Science Foundation of China (No. 81974426 to FZ). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 2837) granted by Institutional Animal Care and Use Committee Board of Huazhong University of Science and Technology, in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Li J, Gu J. Efficacy and safety of ipilimumab for treating advanced melanoma: A systematic review and meta-analysis. J Clin Pharm Ther 2019;44:420-9. [Crossref] [PubMed]
- De Sousa Linhares A, Battin C, Jutz S, et al. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci Rep 2019;9:11472. [Crossref] [PubMed]
- Fei Z, Deng Z, Zhou L, et al. PD-L1 Induces Epithelial-Mesenchymal Transition in Nasopharyngeal Carcinoma Cells Through Activation of the PI3K/AKT Pathway. Oncol Res 2019;27:801-7. [Crossref] [PubMed]
- Wang Y, Wang H, Zhao Q, et al. PD-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med Oncol 2015;32:212. [Crossref] [PubMed]
- Qiu XY, Hu DX, Chen WQ, et al. PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochim Biophys Acta Mol Basis Dis 2018;1864:1754-69. [Crossref] [PubMed]
- Alsuliman A, Colak D, Al-Harazi O, et al. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol Cancer 2015;14:149. [Crossref] [PubMed]
- Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544-8. [Crossref] [PubMed]
- Chakravarthy A, Khan L, Bensler NP, et al. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun 2018;9:4692. [Crossref] [PubMed]
- Tauriello DVF, Palomo-Ponce S, Stork D, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018;554:538-43. [Crossref] [PubMed]
- Batlle E, Massagué J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019;50:924-40. [Crossref] [PubMed]
- Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol 2021;18:9-34. [Crossref] [PubMed]
- Lind H, Gameiro SR, Jochems C, et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J Immunother Cancer 2020;8:e000433. [Crossref] [PubMed]
- Formenti SC, Lee P, Adams S, et al. Focal Irradiation and Systemic TGFβ Blockade in Metastatic Breast Cancer. Clin Cancer Res 2018;24:2493-504. [Crossref] [PubMed]
- Giaccone G, Bazhenova LA, Nemunaitis J, et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer 2015;51:2321-9. [Crossref] [PubMed]
- Funaki S, Shintani Y, Kawamura T, et al. Chemotherapy enhances programmed cell death 1/ligand 1 expression via TGF-β induced epithelial mesenchymal transition in non-small cell lung cancer. Oncol Rep 2017;38:2277-84. [Crossref] [PubMed]
- Ravi R, Noonan KA, Pham V, et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat Commun 2018;9:741. [Crossref] [PubMed]
- Martin CJ, Datta A, Littlefield C, et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci Transl Med 2020;12:eaay8456. [Crossref] [PubMed]
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 2019;20:69-84. [Crossref] [PubMed]
- Jiang Y, Zhan H. Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion. Cancer Lett 2020;468:72-81. [Crossref] [PubMed]
- Wei F, Zhang T, Deng SC, et al. PD-L1 promotes colorectal cancer stem cell expansion by activating HMGA1-dependent signaling pathways. Cancer Lett 2019;450:1-13. [Crossref] [PubMed]
- Kim S, Koh J, Kim MY, et al. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum Pathol 2016;58:7-14. [Crossref] [PubMed]
- Liu C, Yao Z, Wang J, et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ 2020;27:1765-81. [Crossref] [PubMed]
- Mezzadra R, Sun C, Jae LT, et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature 2017;549:106-10. [Crossref] [PubMed]
- Cerezo M, Guemiri R, Druillennec S, et al. Translational control of tumor immune escape via the eIF4F-STAT1-PD-L1 axis in melanoma. Nat Med 2018;24:1877-86. [Crossref] [PubMed]
- Clark CA, Gupta HB, Sareddy G, et al. Tumor-Intrinsic PD-L1 Signals Regulate Cell Growth, Pathogenesis, and Autophagy in Ovarian Cancer and Melanoma. Cancer Res 2016;76:6964-74. [Crossref] [PubMed]
- Shrestha R, Prithviraj P, Bridle KR, et al. Combined Inhibition of TGF-β1-Induced EMT and PD-L1 Silencing Re-Sensitizes Hepatocellular Carcinoma to Sorafenib Treatment. J Clin Med 2021;10:1889. [Crossref] [PubMed]
- Dongre A, Rashidian M, Reinhardt F, et al. Epithelial-to-Mesenchymal Transition Contributes to Immunosuppression in Breast Carcinomas. Cancer Res 2017;77:3982-9. [Crossref] [PubMed]
- Tang MR, Wang YX, Guo S, et al. Prognostic significance of in situ and plasma levels of transforming growth factor β1, -2 and -3 in cutaneous melanoma. Mol Med Rep 2015;11:4508-12. [Crossref] [PubMed]
- Mitra MS, Lancaster K, Adedeji AO, et al. A Potent Pan-TGFβ Neutralizing Monoclonal Antibody Elicits Cardiovascular Toxicity in Mice and Cynomolgus Monkeys. Toxicol Sci 2020;175:24-34. [Crossref] [PubMed]
- Strauss J, Heery CR, Schlom J, et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin Cancer Res 2018;24:1287-95. [Crossref] [PubMed]
- Cheng B, Ding K, Chen P, et al. Anti-PD-L1/TGF-βR fusion protein (SHR-1701) overcomes disrupted lymphocyte recovery-induced resistance to PD-1/PD-L1 inhibitors in lung cancer. Cancer Commun (Lond) 2022;42:17-36. [Crossref] [PubMed]
- Khasraw M, Weller M, Lorente D, et al. Bintrafusp alfa (M7824), a bifunctional fusion protein targeting TGF-β and PD-L1: results from a phase I expansion cohort in patients with recurrent glioblastoma. Neurooncol Adv 2021;3:vdab058. [Crossref] [PubMed]
- Zheng F, Dang J, Zha H, et al. PD-L1 Promotes Self-Renewal and Tumorigenicity of Malignant Melanoma Initiating Cells. Biomed Res Int 2017;2017:1293201. [Crossref] [PubMed]


