
The diagnostic performance of contrast-enhanced ultrasound versus contrast-enhanced computed tomography for pancreatic carcinoma: a systematic review and meta-analysis
Introduction
Pancreatic carcinoma is a highly fatal disease with a low 5-year survival rate of approximately 9% in the United States, and its mortality rate is ranked as the seventh in the world in 2020 (1). The early-stage pancreatic cancer may be cured through surgical resection combined with neoadjuvant and/or adjuvant chemoradiotherapy; however, patients typically present with advanced disease due to lack of or vague symptoms when the cancer is still localized (2). New strategies for screening high-risk patients to detect pancreatic carcinoma at earlier stages are desperately needed. Advances in genomic analysis of human pancreatic tissue and other biospecimens have opened the possibility of DNA-based molecular approaches for early detection of pancreatic cancer, however, some mutations are present at high prevalence in low-grade precancerous lesions with little risk of malignant transformation, a combinatorial approach including additional clinicopathological parameters (such as clinical presentation and radiology) will be required (3).
Contrast-enhanced computed tomography (CECT) is the most frequently used technique for diagnosis and staging of pancreatic carcinoma (4,5). Even in some researches, CECT is regarded as one of the gold standards (2,6). However, CECT is radioactive and the contrast agents are nephrotoxicity, thus it cannot be used in patients with renal insufficiency. Contrast-enhanced ultrasonography (CEUS) is a non-invasive, safe and efficient imaging technique. It can observe the blood flow at the tissue perfusion level with microbubble contrast agents and able to obtain information about tumor perfusion. Several studies have confirmed that CEUS is accurate in the characterization of pancreatic lesions (5,7). Furthermore, CEUS has the advantage of real-time and dynamic imaging; it plays an increasingly important role in diagnosing pancreatic carcinoma. To the authors’ knowledge, CEUS and CECT have not been systematically evaluated in diagnosing pancreatic carcinoma. Thus, the aim of this systematic review and meta-analysis of available literature was to directly compare the diagnostic performance of CEUS and CECT for pancreatic carcinoma, to provide a basis for clinical decision-making. We present the following article in accordance with the PRISMA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-601/rc), and we also registered it in PROSPERO (CRD:42021243566) (8).
Methods
Search strategy
A systematic search was performed in PubMed, EMBASE, Web of Science and Cochrane Library databases, all studies that compared CEUS and CECT in the diagnosis of pancreatic carcinoma from the establishment of the databases to 23rd February 2021 were identified. Search terms included “pancreatic neoplasm”, “CT”, “ultrasonography”, “contrast enhanced”. Details were listed in Table S1. Only English articles were included and references of included articles were crosschecked for relevant studies.
Eligibility criteria and study selection
Two investigators independently reviewed the titles, abstracts, and full texts of the original articles to see whether they were eligible for further quantitative analyses. The inclusion criteria were as follows: (I) study type and index tests: retrospective or prospective studies that used both CEUS and CECT in the diagnosis of pancreatic carcinomas; (II) gold standards: diagnosis of pancreatic carcinomas were confirmed by needle biopsy, surgery and pathology, or alternative imaging modality; (III) studies with reported outcomes that included true positive (TP), true negative (TN), false positive (FP) and false negative (FN), or data from which these values could be calculated.
The exclusion criteria including the following: (I) duplicated studies or those that reported insufficient data; (II) case reports, reviews, letters, abstracts, or editorials. Disagreements were harmonized by consensus. If consensus could not be reached, a third reviewer was consulted.
Data extraction
Two reviewers independently extracted the variables including first author, year of publication, study country, study interval, blinding, patient information (sample size, male/female, mean age), reference standard and the TP, FP, FN, TN of CEUS and CECT from the selected studies.
Quality evaluation
We used the Quality Assessment of Diagnostic Accuracy (QUADAS-2) tool to give quality assessments on all selected studies. The QUADAS-2 scale assesses the publication bias and applicability of the original research from four aspects: patient selection, index test, reference standard and time and flowing (9).
Data statistical analysis
Forest plots were conducted using Review Manager (RevMan; Version 5.4. The Cochrane Collaboration, 2020). The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odds ratio (DOR) with their 95% confidence interval (95% CI) were derived using Stata 16.0 for windows. In this software, we chose a bivariate mixed-effects regression model. Graphs of pre-test probabilities (using the observed median of prevalence from the included studies) against post-test probabilities of CEUS and CECT were created based on the PLR and NLR. In the meanwhile, the chi-squared test (Q test) and inconsistency index (I2) were used to detect interstudy heterogeneity, with P<0.05 and I2≥50% were respectively considered to denote significant heterogeneity. Subgroup analysis was used to explore the heterogeneity source. Forest plots and a summary receiver operating characteristic (SROC) curve were used to illustrate individual and pooled data. The area under the curve (AUC) of SROC was used to calculate the diagnostic accuracy of CEUS and CECT, in which an AUC of 1 was regarded as a perfect test that correctly diagnosed all cases. At last, sensitivity analysis was conducted to assess the robustness of the results.
Subgroup analysis was implemented on the basis of pancreatic lesions’ characteristics. Subgroup 1 included studies that had no limitation on the diameter and location of the pancreatic lesions. Subgroup 2 enrolled the study that researched the small pancreatic carcinoma (≤2 cm), and subgroup 3 included the citation that aimed to compare the diagnostic performance of CEUS and CECT in differentiating carcinomas located in the head of the pancreas.
Meta-regression was used to further explore the causes of the heterogeneity among the studies. The covariates included: (I) study type (prospective vs. retrospective); (II) region (Asia vs. Europe); (III) publication year (before 2010 vs. 2010 and after 2010).
If more than nine studies were included, a Deeks’ funnel plot would be used to assess the publication of bias.
Results
Search results
A total of 1,227 records were identified through the databases, of which 211 articles were excluded for duplication. After reading the title and abstracts, 953 reports that did not meet the inclusion criteria were discarded. Sixty-three articles were assessed by the full text and seven studies were eventually included for meta-analysis. Figure 1 shows the details of the process used to select the included studies.
Study characteristics and quality assessment
A total of 588 patients were included among the seven citations, of which 364 patients were diagnosed as pancreatic carcinoma. We summarized the characteristics of each study in Table 1. Two studies (10,11) respectively restricted the tumor size and location of the included sample, which may induce heterogeneity. The QUADAS-2 tool in Review Manager 5.4 indicated the quality of the selected studies. Because none of the seven articles (4,5,10-14) mentioned whether the gold standard was performed blind to the images of CEUS and CECT, the risk of bias of the reference standard of the seven items were all unclear. Four studies (11-14) had no information about the period between the index test and the reference standard. Studies published before 2010 (12-14) used Levovist as the contrast agent of CEUS which has been replaced by SonoVue or Sonozoid; thus, we gave high concern of its applicability. Detailed assessment on the quality of the studies was shown in Figure 2.
Table 1
| Author [year] | Country | Study type | Study interval | Imaging | Patient information | Diagnostic standard | Diagnostic criteria | Microbubble contrast agents of CEUS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | Male/female | Age (years) | CEUS | CECT | |||||||
| Tanaka (10) [2020] | Japan | Retro | 2007 to 2017 | Blinded | 200 | 108/92 | 65.5 | Histopathology and clinical course | Hypo-enhancement | Hypo-enhancement | Sonazoid |
| Fan (5) [2013] | China | Retro | Jan 2009 to Sep 2012 | Blinded | 90 | 53/37 | 55.1 | Histopathology and clinical course | Hypo-enhancement | Hypo-enhancement | Sono Vue |
| Vasile (4) [2012] | Romania | Pro | Mar 2009 to Feb 2012 | Blinded | 76 | 38/38 | 59.5 | Histopathology | Hypovascular pattern | Hypovascular pattern | Sono Vue |
| Grossjohann (11) [2010] | Denmark | Retro | Feb 2005 to Dec 2007 | Unblinded | 49 | 26/23 | 66 | Histopathology | Hypoechogenicity | NA | Sono Vue |
| Okamoto (12) [2006] | Japan | Pro | Sep 2002 to Jun 2005 | Blinded | 62 | 43/19 | 61 | Histopathology and clinical course |
Hypo-enhancement | Hypo-enhancement | Levovist |
| Sofuni (13) [2005] | Japan | Pro | Sep 2000 to Dec 2002 | Blinded | 46 | NA | NA | Histopathology | Hypovascular and hypoperfusion pattern | Hypovascular pattern | Levovist |
| Kitano (14) [2003] | Japan | Pro | Mar 2001 to Aug 2003 | Blinded | 65 | NA | NA | Histopathology and clinical course | Hypovascular pattern | Hypovascular pattern | Levovist |
CEUS, contrast-enhanced ultrasonography; CECT, contrast-enhanced computed tomography; Retro, retrospective study; Pro, prospective study; NA, not available.
Heterogeneity tests
The chi-squared test and inconsistency index of sensitivity and specificity for CEUS (I2=58.55%; P=0.02 and I2=78.77%; P=0.00) and CECT (I2=67.15%; P=0.01 and I2=72.15%; P=0.00) revealed significant heterogeneity among the included seven articles. However, in subgroup 1, the I2 values of the specificity, positive diagnostic ratio and the diagnostic score of CEUS and CECT showed no heterogeneity (I2=0%); for sensitivity, the I2 values of CEUS and CECT were 31.96% (P=0.21) and 49.45% (P=0.09), respectively. Thus, tumor characteristic was one of the causes of heterogeneity. For CECT, regional difference may be a significant cause of heterogeneity (P=0.00). It seemed that European studies had higher sensitivity than that of Asian articles, in addition, study type was strongly associated with the heterogeneity of specificity (P=0.00). The specificity of retrospective studies was higher than that of prospective studies. The meta-regression analysis results are summarized in Table 2.
Table 2
| Variables | Sensitivity (95% CI) | P value | Specificity (95% CI) | P value |
|---|---|---|---|---|
| CEUS | ||||
| Study type | 0.15 | 0.08 | ||
| Prospective (n=3) | 0.93 (0.87–0.98) | 0.77 (0.62–0.92) | ||
| Retrospective (n=4) | 0.89 (0.83–0.95) | 0.87 (0.76–0.98) | ||
| Region | 0.45 | 0.46 | ||
| Asia (n=5) | 0.93 (0.89–0.97) | 0.81 (0.69–0.94) | ||
| Europe (n=2) | 0.84 (0.74–0.95) | 0.86 (0.69–1.00) | ||
| Publication year | 0.06 | 0.25 | ||
| Before 2010 (n=3) | 0.90 (0.84–0.97) | 0.81 (0.68–0.93) | ||
| 2010 and after 2010 (n=4) | 0.91 (0.85–0.98) | 0.88 (0.74–1.00) | ||
| CECT | ||||
| Study type | 0.25 | 0.00 | ||
| Prospective (n=3) | 0.89 (0.81–0.98) | 0.81 (0.73–0.88) | ||
| Retrospective (n=4) | 0.86 (0.78–0.94 | 0.92 (0.85–0.99) | ||
| Region | 0.00 | 0.36 | ||
| Asia (n=5) | 0.85 (0.79–0.91) | 0.89 (0.76–1.00) | ||
| Europe (n=2) | 0.92 (0.86–0.99) | 0.77 (0.50–1.00) | ||
| Publication year | 0.29 | 0.09 | ||
| Before 2010 (n=3) | 0.90 (0.83–0.96) | 0.83 (0.75–0.90) | ||
| 2010 and after 2010 (n=4) | 0.85 (0.75–0.94) | 0.97 (0.91–1.00) |
CI, confidence interval; CEUS, contrast-enhanced ultrasonography; CECT, contrast-enhanced computed tomography.
Meta-analysis of diagnostic performance
The overall sensitivity (0.91; 95% CI: 0.85–0.94), specificity (0.83; 95% CI: 0.70–0.91), AUC (0.94), PLR (5.23; 95% CI: 3.00–9.10), NLR (0.11; 95% CI: 0.07–0.17) and DOR (46.91; 95% CI: 25.13–87.55) supported a great capability of CEUS to distinguish pancreatic carcinoma. The specificity (0.87; 95% CI: 0.73–0.94), PLR (6.55; 95% CI: 3.07–13.99) and NLR (0.14; 95% CI: 0.09–0.23) of CECT performed better than that of CEUS; but the sensitivity (0.88; 95% CI: 0.81–0.92), AUC (0.93) and DOR (45.58; 95% CI: 16.72–124.24) were a little lower. The forest plots and SROC were displayed in Figures 3,4.
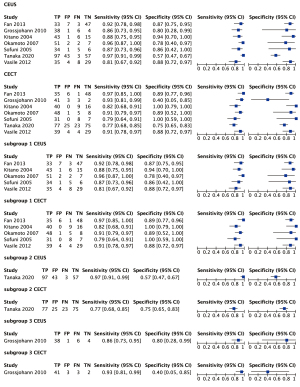
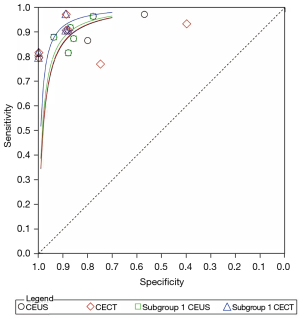
In subgroup 1, the specificity, AUC and DOR of CECT improved compared with the overall group and manifested better than CEUS. Subgroup 2 displayed high sensitivity of CEUS (100%) for characterizing small cancers (≤2 cm) but low sensitivity of CECT (76.7%). Subgroup 3 showed an unusual low specificity of CECT for the diagnosis of pancreatic carcinoma. The sensitivity of CEUS in subgroup 1 decreased compared to the overall group. Details were listed in Table 3. The above results suggested that CEUS may be good at diagnosing lesions in the head of the pancreas or with diameters less than 2 cm.
Table 3
| Methods | Sensitivity (95% CI) | Specificity (95% CI) | AUC | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | |
|---|---|---|---|---|---|---|---|
| Overall | CEUS | 0.91 (0.85–0.94) | 0.83 (0.70–0.91) | 0.94 | 5.23 (3.00–9.10) | 0.11 (0.07–0.17) | 46.91 (25.13–87.55) |
| CECT | 0.88 (0.81–0.92) | 0.87 (0.73–0.94) | 0.93 | 6.55 (3.07–13.99) | 0.14 (0.09–0.23) | 45.58 (16.72–124.24) | |
| Subgroup 1 | CEUS | 0.89 (0.84–0.93) | 0.87 (0.80–0.92) | 0.92 | 7.06 (4.38–11.4) | 0.12 (0.08–0.19) | 55.59 (28.11–117.99) |
| CECT | 0.88 (0.81–0.93) | 0.92 (0.82–0.97) | 0.96 | 11.67 (4.85–28.09) | 0.12 (0.08–0.21) | 93.74 (33.63–261.33) |
AUC, the area under the curve of summary receiver operating characteristics; NLR, negative likelihood ratio; PLR, positive likelihood ratio; DOR, diagnostic odds ratio; 95% CI, 95% confidence intervals; CEUS, contrast-enhanced ultrasonography; CECT, contrast-enhanced computed tomography.
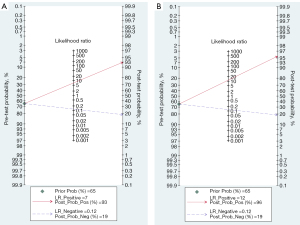
Heterogeneity in subgroup 1 could be ignored, thus we calculated the post-test probability of CEUS and CECT based on its PLR and NLR. The mean pre-test probability was 64.9% among the included people (patients suspected of pancreatic cancer or patients with hypoechoic pancreatic lesions on ultrasound). At this pre-test probability, the post-test probability of pancreatic carcinoma for CEUS and CECT were calculated in Figure 5. This meant that 93% and 96% of people with pancreatic lesions who have positive CEUS and CECT results may potentially have pancreatic carcinoma separately.
We omitted one study at a time for sensitivity analysis and the result indicated the synthesized data were robust.
Publication bias
According to the Cochrane handbook, when more than nine studies are included in meta-analysis, funnel plots should be used to detect publication bias. We only included seven articles in our meta-analysis, so we did not construct this process.
Discussion
For silent onset and rapid invasiveness, patients with pancreatic carcinoma are usually diagnosed at advanced stages and tend to have a poor prognosis, thus, early detection and accurate diagnosis of pancreatic cancers are of importance. Traditional transabdominal ultrasonography (US) is usually regarded as the first-line examination to detect pancreatic diseases when no symptoms are present. Malignant pancreatic tumors often appear as hypoechoic lesions with irregular margins, nevertheless, many other pancreatic diseases, such as pancreatitis, can also manifest similarly (10). CECT and magnetic resonance imaging (MRI) are commonly utilized for the workup of pancreatic masses (15). But due to the fixed time point of scanning, CECT cannot capture the transient enhancement and is limited in some exceptive lesions (5). MRI has higher soft-tissue contrast than CECT and should be considered to be used after basic examination (16). Other methods used for definite diagnosis such as EUS-guided FNA (EUS-FNA) are invasive and not suitable for extensive screening of high-risk groups.
Recently, CEUS has been successfully applied in the imaging of organ-specific diseases thanks to the dynamic observation of the contrast-enhanced phases after the injection of a purely intravascular contrast agent (17). The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) (18) recommends that CEUS can be used to reliably characterize ductal adenocarcinoma in solid pancreatic lesions detected on ultrasound. Wang et al. (19) found that the diagnostic accuracy of CEUS was not significantly different from that of enhanced CT for 146 cases of solid pancreatic lesions. Several meta-analyses (17,20) reported that CEUS had a good diagnostic ability and accuracy for benign and malignant pancreatic neoplasms, but they did not directly compare the results of CEUS with CECT which was broadly used for diagnosing pancreatic neoplasms in clinical practice. We conducted this meta-analysis to further compare the diagnostic performance of CEUS with that of CECT for the detection of pancreatic carcinoma. In order to strengthen comparability, we set stringent inclusion criteria, and only studies regarded both CEUS and CECT as index tests were included. Seven studies with 588 patients were eventually analyzed. To the authors’ knowledge, this is the first meta-analysis for this purpose.
The overall results of CEUS corresponded with the existing meta-analyses and multicenter study (7,17,20,21). The pooled sensitivity (0.88; 95% CI: 0.81–0.92), specificity (0.87; 95% CI: 0.73–0.94) of CECT were close to the result of a large meta-analysis of 3,567 patients with PDAC (22). In our research, CEUS seems to have higher sensitivity but lower specificity compared with CECT, as for AUC, DOR and post-test probability, the two techniques perform similarly.
Tanaka et al. (10) (subgroup 2) aimed to evaluate the effectiveness of CEUS for the characterization of small and early-stage pancreatic adenocarcinoma. In this study, the sensitivity of CEUS (100%) for characterizing small cancers (≤2 cm) was significantly higher than the pooled sensitivity of CEUS in subgroup 1 for diagnosing all kinds of carcinomas; while the sensitivity of CECT was much lower (76.7%). Studies (23-25) showed that the sensitivity of CECT was not sufficiently high (72–77%) for small pancreatic adenocarcinoma. This was possibly due to the uncommon occurrence of visually iso-attenuating pancreatic adenocarcinoma whose prevalence may have relationships with the tumor size and cellular differentiation (23,26). Yoon et al. (23) demonstrated that iso-attenuating pancreatic cancers were more commonly observed among the 2 cm or smaller tumors (16 of 59, 27%) than among the 2–3 cm tumors (12 of 93, 13%), thus small pancreatic carcinomas were more likely to be overlooked in CECT. On the contrary, CEUS seems to have an advantage over CECT in diagnosing small pancreatic carcinomas. D’Onofrio et al. (25) compared CEUS and multi-detector computed tomography (MDCT) features of pancreatic adenocarcinoma in relation to tumor size, they found that for lesions smaller than 2 cm, CEUS had 100% sensitivity, while sensitivity of MDCT was only 73.33%; for lesions larger than 4 cm, CEUS had a sensitivity of 87.88% while MDCT had a sensitivity of 100%, thus they draw a conclusion that CEUS sensitivity seemed to be higher for small and medium lesions, while MDCT sensitivity was higher for large lesions. Considering the importance of identifying high-grade dysplastic neoplastic lesions and early cancer to enable timely resection and potentially improve survival (27), CEUS is worthy of further studying.
Of the 7 enrolled studies, the study of Grossjohann et al. (11) researched the tumors in the head of pancreas and was listed in subgroup 3. They reported low specificity of 40% in CECT, which was quite different with the other included studies. Through further analysis, we found that they enrolled 49 consecutive patients with pancreatic head lesions, in which 44 patients had pancreatic adenocarcinoma and 5 had chronic pancreatitis. Chronic pancreatitis is a fibroinflammatory syndrome in which repetitive episodes of pancreatic inflammation leads to extensive fibrotic tissue replacement (28), it sometimes may mimic pancreatic adenocarcinoma in imaging; in addition, as mentioned above, iso-attenuating pancreatic carcinomas may be misdiagnosed as pancreatitis. Kang et al. (29) analyzed the factors associated with missed and misinterpreted cases of pancreatic ductal adenocarcinoma, they found that a significant number of missed tumors were <2 cm (45/107, 42%), iso-attenuating on CT (32/72, 44%) or non-contour deforming (44/107, 41%), and most (29/49, 59%) misinterpreted examinations were reported as uncomplicated pancreatitis. Therefore, given that differentiation between pancreatic adenocarcinoma and focal chronic pancreatitis remains a challenge in CECT (30), the low specificity of CECT for depicting pancreatic carcinoma in this study seems reasonable; besides, the low amount of disease negative patients may also be a factor causing the low specificity. This also resulted in the low combined specificity in the total group. Surprisingly, the specificity of CEUS in this study was 80% which indicated CEUS may be an excellent diagnostic technique for mass-forming pancreatitis. Using iso-enhancement or iso-enhancement with focal hypo-enhancement in both the early and late phases as diagnostic criteria, Wang et al. (31) found that the diagnostic sensitivity, specificity, and accuracy of CEUS for diagnosing focal pancreatitis were 72.0%, 95.5%, and 91.2%, respectively. The study of Fan (5) also supported the result that CEUS performed well in the diagnosis of pancreatitis. But it’s as yet a challenge to explain the reason for the result. Better differentiation of vascularization by CEUS and the poor soft-tissue contrast between focal mass-like pancreatitis lesions and the normal pancreatic parenchyma by CECT may play a role (16,32).
Getting rid of the two studies in subgroup 2 and 3, the sensitivity of CEUS in subgroup 1 decreased and the specificity of CECT increased compared with the overall group, thus the AUC of CECT turned to be better than that of CEUS. The above conclusions show that the combined use of CEUS and CECT can improve the diagnostic efficiency and make it easier to achieve the purpose of early diagnosis of malignant pancreatic tumors.
There was still some heterogeneity among the sensitivity of CEUS and CECT in subgroup 1. The results of meta-regression showed region was related to the heterogeneity in sensitivity of CECT. The geographic distribution and the different characteristics of tumor among ethnic groups might induce variations. In our analysis, studies in Europe had higher sensitivity for CECT than that in Asia, but it was based on a small quantity of researches. For CEUS, it is quite operator dependent, sometimes the interpretation of the images may be subjective; in addition, affected by the intestinal gas and thickness of abdominal wall, patients with inadequate quality are excluded in the original study. Thus, the inter-institutional biases may be an important source of the high inconsistency index of our meta-analysis and cannot be ignored. Double contrast-enhanced ultrasonography (DCEUS) which is performed with both luminal and intravascular contrast agents may be a promising tool to alleviate the influence of stomach gas (33).
There are some limitations in our study. First, only 7 eligible researches were included, and this small sample size may limit the power of the data analysis or the generalizability of the study findings. Second, we only enrolled literatures published in the English language, which may have resulted in some studies being omitted. Moreover, Grossjohann et al. regarded 64-CT as one of the standard diagnostic method of pancreatic carcinoma, which may also influence our results (11). At last, due to the rapid development of technology, the slice thickness of some enrolled articles nowadays is not a standard thickness for diagnosing pancreatic tumor.
Conclusions
Our meta-analysis showed both CEUS and CECT had good performance in the diagnosis of pancreatic carcinoma, whereas compared to CECT, CEUS had high sensitivity for lesions smaller than 2 cm or in early stage; in addition, CEUS had an advantage over CECT for diagnosing chronic pancreatitis. However, our results were based on a small number of retrospective or prospective studies, many of which were performed in Japan. In addition, due to the high inconsistency index, the inter-institutional difference cannot be ignored. Further prospective and multi-center studies are therefore needed before generalization of this conclusion.
Acknowledgments
Funding: This paper is an independent research funded by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-601/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-601/coif). All authors report that this study was funded by the National Natural Science Foundation of China (No. 81873902); the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2016-I2M-3-005); and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS) (No. 2020-I2M-C&T-B-039). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Yang J, Xu R, Wang C, et al. Early screening and diagnosis strategies of pancreatic cancer: a comprehensive review. Cancer Commun (Lond) 2021;41:1257-74. [Crossref] [PubMed]
- Singhi AD, Wood LD. Early detection of pancreatic cancer using DNA-based molecular approaches. Nat Rev Gastroenterol Hepatol 2021;18:457-68. [Crossref] [PubMed]
- Vasile TA, Feier D, Socaciu M, et al. Contrast enhanced ultrasound and computer tomography diagnosis of solid and mixed pancreatic tumors - analysis of confounders. J Gastrointestin Liver Dis 2012;21:285-92. [PubMed]
- Fan Z, Li Y, Yan K, et al. Application of contrast-enhanced ultrasound in the diagnosis of solid pancreatic lesions--a comparison of conventional ultrasound and contrast-enhanced CT. Eur J Radiol 2013;82:1385-90. [Crossref] [PubMed]
- Ardelean M, Sirli R, Sporea I, et al. The value of contrast-enhanced ultrasound in the characterization of vascular pattern of solid pancreatic lesions. Med Ultrason 2015;17:16-21. [Crossref] [PubMed]
- D'Onofrio M, Barbi E, Dietrich CF, et al. Pancreatic multicenter ultrasound study (PAMUS). Eur J Radiol 2012;81:630-8. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Tanaka S, Fukuda J, Nakao M, et al. Effectiveness of Contrast-Enhanced Ultrasonography for the Characterization of Small and Early Stage Pancreatic Adenocarcinoma. Ultrasound Med Biol 2020;46:2245-53. [Crossref] [PubMed]
- Grossjohann HS, Rappeport ED, Jensen C, et al. Usefulness of contrast-enhanced transabdominal ultrasound for tumor classification and tumor staging in the pancreatic head. Scand J Gastroenterol 2010;45:917-24. [Crossref] [PubMed]
- Okamoto Y, Kawamoto H, Takaki A, et al. Contrast-enhanced ultrasonography depicts small tumor vessels for the evaluation of pancreatic tumors. Eur J Radiol 2007;61:163-9. [Crossref] [PubMed]
- Sofuni A, Iijima H, Moriyasu F, et al. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol 2005;40:518-25. [Crossref] [PubMed]
- Kitano M, Kudo M, Maekawa K, et al. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut 2004;53:854-9. [Crossref] [PubMed]
- Elsherif SB, Virarkar M, Javadi S, et al. Pancreatitis and PDAC: association and differentiation. Abdom Radiol (NY) 2020;45:1324-37. [Crossref] [PubMed]
- Ha J, Choi SH, Byun JH, et al. Meta-analysis of CT and MRI for differentiation of autoimmune pancreatitis from pancreatic adenocarcinoma. Eur Radiol 2021;31:3427-38. [Crossref] [PubMed]
- D'Onofrio M, Biagioli E, Gerardi C, et al. Diagnostic performance of contrast-enhanced ultrasound (CEUS) and contrast-enhanced endoscopic ultrasound (ECEUS) for the differentiation of pancreatic lesions: a systematic review and meta-analysis. Ultraschall Med 2014;35:515-21. [Crossref] [PubMed]
- Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med 2018;39:e2-e44. [Crossref] [PubMed]
- Wang Y, Li G, Yan K, et al. Clinical value of contrast-enhanced ultrasound enhancement patterns for differentiating solid pancreatic lesions. Eur Radiol. 2022;32:2060-9. [Crossref] [PubMed]
- Li XZ, Song J, Sun ZX, et al. Diagnostic performance of contrast-enhanced ultrasound for pancreatic neoplasms: A systematic review and meta-analysis. Dig Liver Dis 2018;50:132-8. [Crossref] [PubMed]
- Ran L, Zhao W, Zhao Y, et al. Value of contrast-enhanced ultrasound in differential diagnosis of solid lesions of pancreas (SLP): A systematic review and a meta-analysis. Medicine (Baltimore) 2017;96:e7463. [Crossref] [PubMed]
- Toft J, Hadden WJ, Laurence JM, et al. Imaging modalities in the diagnosis of pancreatic adenocarcinoma: A systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracy. Eur J Radiol 2017;92:17-23. [Crossref] [PubMed]
- Yoon SH, Lee JM, Cho JY, et al. Small (≤ 20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 2011;259:442-52. [Crossref] [PubMed]
- Bronstein YL, Loyer EM, Kaur H, et al. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am J Roentgenol 2004;182:619-23. [Crossref] [PubMed]
- D'Onofrio M, Crosara S, Signorini M, et al. Comparison between CT and CEUS in the diagnosis of pancreatic adenocarcinoma. Ultraschall Med 2013;34:377-81. [PubMed]
- Kim JH, Park SH, Yu ES, et al. Visually iso-attenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 2010;257:87-96. [Crossref] [PubMed]
- Overbeek KA, Goggins MG, Dbouk M, et al. Timeline of Development of Pancreatic Cancer and Implications for Successful Early Detection in High-Risk Individuals. Gastroenterology 2022;162:772-785.e4. [Crossref] [PubMed]
- Beyer G, Habtezion A, Werner J, et al. Chronic pancreatitis. Lancet 2020;396:499-512. [Crossref] [PubMed]
- Kang JD, Clarke SE, Costa AF. Factors associated with missed and misinterpreted cases of pancreatic ductal adenocarcinoma. Eur Radiol 2021;31:2422-32. [Crossref] [PubMed]
- Song P, Yan JY, Wang Y, et al. Value of multi-detector computed tomography during intra-arterial infusion of contrast medium for locating insulinomas. J Int Med Res 2020;48:300060519889432. [Crossref] [PubMed]
- Wang Y, Yan K, Fan Z, et al. Clinical Value of Contrast-Enhanced Ultrasound Enhancement Patterns for Differentiating Focal Pancreatitis From Pancreatic Carcinoma: A Comparison Study With Conventional Ultrasound. J Ultrasound Med 2018;37:551-9. [Crossref] [PubMed]
- D'Onofrio M, Malagò R, Zamboni G, et al. Contrast-enhanced ultrasonography better identifies pancreatic tumor vascularization than helical CT. Pancreatology 2005;5:398-402. [Crossref] [PubMed]
- Zhu W, Mai G, Zhou X, et al. Double contrast-enhanced ultrasound improves the detection and localization of occult lesions in the pancreatic tail: a initial experience report. Abdom Radiol (NY) 2019;44:559-67. [Crossref] [PubMed]




