
The association of tumor diameter with lymph node metastasis and recurrence in patients with endometrial cancer: a systematic review and meta-analysis
Highlight box
Key findings
• The TD of EC patients is closely related to LNM and recurrence. TD >2 cm can be used as a reference index to predict LNM of EC patients.
What is known and what is new?
• As an easily available indicator, TD has been reported to have a certain predictive effect on LNM and recurrence of EC patients, but the relationship between TD and LNM and recurrence of EC is still controversial.
• This study resolves the controversy over the relationship between TD and LNM and recurrence in patients with EC.
What is the implication, and what should change now?
• TD is easily measured during surgery, so that clinicians using TD to determine a complete surgical staging could to some extent reduce unnecessary LND and avoid secondary surgery, while estimating the risk of recurrence based on TD can also lead to better treatment outcomes for the patient.
Introduction
Endometrial cancer (EC) has been shown to be the most prevalent gynecological malignancy in developed countries, with a gradually increasing morbidity in developing countries. Abnormal uterine bleeding is one of the main clinical manifestations of EC, accounting for 75–90% of the cases, and the most prevalent risk factors include obesity, fat-rich diet, early menarche, type 2 diabetes, lynch syndrome, age over 55 years old, sterility and infertility, delayed menopause, concomitance with anovulatory diseases or functional ovarian tumor, and long-term medication history of single estrogen or tamoxifen (1-5). Pelvic and para-aortic lymph node dissection (LND) could be selectively added in the staging surgery for EC resting on the existence of high-risk factors for lymph node (LN) involvement (5,6). It is reported that the incidence of pelvic LN (PLN) or para-aortic LN (PALN) involvement ranges from 5% to 20% (7). Chemoradiotherapy could be considered based on the cancer stage of the patient. Lymph node metastasis (LNM) is the main spreading pattern of EC, and is closely related to patient prognosis. The recurrence rate of EC in LNM patients far exceeds that in non-LNM patients (48% vs. 8%) (8). Additionally, it is reported that the 5-year disease-free survival (DFS) is 90% in non-LNM patients, and 75% in those with pelvic LNM (PLNM). The occurrence of PLNM indicates poorer prognosis, with a 5-year DFS of only 38% (9,10). Therefore, the status of LNs has an important effect on the prognosis of EC patients.
It remains controversial whether LND should be performed during EC surgery, as well as the scope of dissection. Research by Bougherara et al. (11), has demonstrated that implementing LND could result in increased surgical time, perioperative bleeding, and injury to nerves, vessels, and ureter, as well as increased incidence of postoperative complications such as lymphedema, lymphocele, ileus, and lower limb vein thrombosis. Given this situation, some scholars have formulated different standards to assess the risk of LNM in EC patients. The Mayo clinic has developed an algorithm for EC treatment, that is, the “Mayo standard”, which defines LNM low-risk EC patients as: endometrioid EC with the International Federation of Gynecology and Obstetrics (FIGO) grade 1 or 2, muscular infiltration (MI) <50%, and tumor diameter (TD) ≤2 cm. Other EC patients would be defined as high-risk. LND would be no longer considered for low-risk EC patients, whereas systematic LND up to the renal vein level should be performed for those with high risk (11). Though LN involvement accounts for approximately 15% of the endometrioid EC patients, 75% of the female patients need systematic LND when applying the Mayo standard (12). Therefore, Vargas et al. suggest that the definition of LNM low-risk EC patients in the Mayo standard could be modified as follows: endometrioid EC with pathological grade 1 and MI <50%, EC with pathological grade 2 and TD <3 cm, or EC with pathological grade 3 and without MI (13). A Gynecologic Oncology Group Study has proposed a Milwaukee model which defines the LNM low-risk patients as: TD ≤5 cm with MI ≤33% (10). It can be noticed that there is controversy among researchers over cut-off value of TD (whether should be 2, 3, or 5 cm). This controversy may be related to the small sample size included in the study, different ways of measuring TD, etc.
In addition, some scholars have developed different criteria for evaluating the prognosis of patients to formulate different treatment plans, in which the risk of recurrence is included. Characteristics of low-risk EC are defined, according to European Society for Medical Oncology (ESMO) guideline, as endometrioid carcinoma with MI ≤50% and FIGO grade I or II (14), which was modified by Bendifallah et al. in 2014 (15). The World Health Organization (WHO) has included lymphatic vascular space invasion (LVSI) in the model (ESMO-modified classification) (15). Keys et al. grade the risk in EC patients based on their age, histological classification, cancer grade, lymphatic invasion, and depth of basal invasion, so as to determine whether adjuvant treatment should be considered (GOG-99 standard) (16). The modified ESMO and GOG-99 were introduced for decision-making of adjuvant therapy in EC patients, yet TD remains unincluded, which might be due to that its effect is still under investigation (17). Some studies indicate an association between TD and LNM. Some researchers have proposed that TD might be associated with the recurrence of EC (18,19). TD is easily measured during surgery, so that clinicians using TD to determine a complete surgical staging could to some extent reduce unnecessary LND and avoid secondary surgery (20), while estimating the risk of recurrence based on TD can also lead to better treatment outcomes for the patient.
It can be gleaned from the studies mentioned above that TD is closely related to LNM and recurrence in EC patients, whereas the association of the TD with LNM and recurrence is still controversial. Therefore, this systematic review and meta-analysis aimed to evaluate the association of TD with LNM and recurrence in EC patients, so as to provide more evidence for clinical EC treatment. We present the following article in accordance with the MOOSE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2595/rc).
Methods
Literature search
The databases of PubMed, Embase, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP, and Wanfang were searched, from inception to 27 October 2022, for studies regarding the association of EC diameter/original lesion area with the risk of LNM and recurrence. The search strategy and items were designed according to the Cochrane handbook and search rules of each database, with language restricted to Chinese and English.
Inclusion and exclusion criteria
Inclusion criteria
Patients who were diagnosed histologically with single EC before surgery; study that reported TD or data related to LNM and recurrence; outcome measures included LNM or recurrence; types of study: observational study (cohort study/case-control study).
Exclusion criteria
Animal study, study with data or full-text unavailable, literature review, meta-analysis, case report, monograph, ongoing clinical trial, and study with participants less than 20.
Data extraction
All retrieved articles were classified by two reviewers (Ruifang Fu and Xiaohan Yu) according to the data required. All the articles were divided into a LNM group and a recurrence group based on the following aspects:
- LNM: first author’s surname, country of origin, year of publication, pathological grade, FIGO stage, type of study, number of patients (sample size), age, odds ratio (OR) and 95% confidence interval (CI) about the association of TD and the risk of LNM (the OR value obtained from the multivariate analysis is first extracted; the results of univariate analysis were extracted for articles without the results of multivariate analysis), LNM metastatic site and cut-off value (cm).
- Recurrence: first author’s surname, country of origin, year of publication, pathological grade, FIGO stage, type of study, number of patients (sample size), age, OR and 95% CI about the association of TD and the risk of recurrence (the OR value obtained from the multivariate analysis is first extracted; the results of univariate analysis were extracted for articles without the results of multivariate analysis), and cut-off value (cm).
Quality assessment
Quality of included cohort studies were assessed using Newcastle-Ottawa Scale (NOS) (21). All studies included in this study were retrospective cohort studies, so all of them used NOS for quality assessment. The NOS contains 2 forms designed respectively for cohort study and case-control study. The form of cohort study involves 3 domains with 8 items: selection, comparability, and outcome. The form of case-control study also involves 3 domains with 8 items: selection, comparability, and exposure. It could be scored 1 point if meeting the requirements, with a total score for 9. The higher the score, the higher the quality of the study.
Statistical analysis
All data analyses were processed using Stata 15.0 software (StataCorp., College Station, TX, USA). OR and 95% CI were directly extracted from each publication to evaluate the association of TD and the risk of LNM and recurrence in EC patients. The OR value obtained from the multivariate analysis is first extracted from each study (all the multivariate analysis variables were statistically significant variables in the univariate analysis). The results of univariate analysis were extracted for articles without the results of multivariate analysis. The Cochran Q and I2 statistical methods were applied to evaluate the heterogeneity among included studies. A P≥0.1 with an I2<50% would indicate no significant heterogeneity among the studies, and fixed-effect model would be applied. Otherwise, P<0.1 and I2≥50%, significant heterogeneity would be considered, and random-effect model would be applied. Sensitivity analysis was carried out to assess the influence of each individual study on the pooled results by sequentially excluding each study and subgroup analysis would be performed to identify the source of heterogeneity. Potential publication bias was evaluated by Egger’s test with funnel plots. Bilateral P value<0.05 was regarded statistically significant.
Results
Study selection
There were 6,811 articles identified, and 1,520 duplicated or ineligible articles were removed. Titles and abstracts of the remaining articles were browsed, in strict accordance with the inclusion and exclusion criteria, for initial screening. A total of 69 studies were finally included after reading the full-texts, in which 48 studies focused on LNM, 25 studies on recurrence, and 4 on the both. The study selection process is presented in Figure 1.
Characteristics of included studies
A total of 69 retrospective cohort studies were included. Detailed characteristics of included studies are presented in Tables 1,2.
Table 1
| Author | Year | Country/region | Pathological grade | FIGO stage | Type of study | Sample size | Age (years)* | Univariate or multivariate | Metastatic site | Cut-off value (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Li X (22) | 2021 | China | G1–G3 | Not provided | RC | 63,836 | 62.41±11.62 | Multivariate | Full range | 2, 5, 10 |
| Meydanli MM (23) | 2019 | Turkey | G1–G3 | I–IV | RC | 966 | 58 [31–84] | Multivariate | Full range | 4 |
| Matsushita C (24) | 2019 | Japan | G1–G3 | I–IV | RC | 185 | 57 [33–78] | Multivariate | Full range | 2 |
| Dong Y (25) | 2019 | China | G1–G3 | I–II | RC | 1,427 | 60 [35–77] | Univariate | Full range | 2 |
| Nasioudis D (26) | 2019 | USA | G1–G3 | IA, IB | RC | 14,398 | 63.0 | Univariate | Abdominal aorta | 2 |
| Günakan E (27) | 2019 | Turkey | Not provided | I–IV | RC | 762 | 59.1 | Univariate | Full range | 2 |
| Yildirim N (28) | 2018 | Turkey | G1–G4 | I–IV | RC | 278 | 60.1±9.8 | Univariate | Full range | 2 |
| Toptaş T (29) | 2017 | Turkey | G1–G3 | Not provided | RC | 128 | 59.3±11.2 | Multivariate | Full range | 3 |
| Sari ME (7) | 2017 | Turkey | G1–G4 | I–IV | RC | 641 | 59 [28–85] | Univariate | Abdominal aorta | 2, 4 |
| Lucic N (30) | 2017 | Serbia | G1–G3 | Not provided | RC | 221 | 60 [31–88] | Univariate | Full range | 2 |
| Boyraz G (31) | 2017 | Turkey | G1–G2 | IA | RC | 191 | 57.8 | Univariate | Full range | 2 |
| Cox Bauer CM (32) | 2016 | USA | G1–G3 | I–III | RC | 737 | 62.8 | Univariate | Full range | 2, 3, 4, 5 |
| Canlorbe G (33) | 2016 | France | G1–G3 | I–II | RC | 633 | 65.6 [58.0–72.3] | Univariate | Full range | 2, 3.5 |
| Bourgioti C (34) | 2016 | Hellenic | G1–G3 | I–IV | RC | 105 | 59.8±12.6 | Univariate | Full range | 4 |
| Cetinkaya K (35) | 2016 | Turkey | G1–G3 | I–III | RC | 268 | 58.6 [27–80] | Univariate | Full range | 2 |
| Bendifallah S (36) | 2015 | France | G1–G3 | I\IIIC | RC | 523 | 64.9 [33–98] | Univariate | Full range | 1 |
| Bendifallah S (37) | 2015 | France | Not provided | I–III | RC | 457 | 66.4 [31–98] | Univariate | Full range | 1.5 |
| Rathod PS (38) | 2014 | India | G1–G3 | IA–IIIC2 | RC | 52 | 58.3 [31–76] | Univariate | Abdominal aorta and pelvic cavity | 2 |
| Mahdi H (39) | 2015 | USA | G1–G4 | I | RC | 19,692 | 62.1 | Univariate | Full range | 2, 5 |
| Gilani S (40) | 2014 | USA | G1–G3 | Not provided | RC | 207 | 62.29±10.9 | Univariate | Full range | 2 |
| AlHilli MM (41) | 2013 | USA | G1–G3 | I–II | RC | 883 | 63.9 | Univariate | Full range | 2 |
| Shah C (20) | 2005 | USA | G1–G3 | I–IV | RC | 345 | Not provided | Multivariate | Full range | 1 |
| Watanabe M (42) | 2003 | Japan | G1–G2 | IA–IIIC | RC | 107 | 54 [29–79] | Univariate | Full range | 2 |
| Cheng WF (43) | 1998 | China | G1–G3 | Not provided | RC | 42 | 52.3 [25–78] | Univariate | Full range | 2.5 |
| Wu SW (44) | 2021 | China | G1–G3 | I–III | RC | 1,346 | 60.0 | Multivariate | Full range | 2 |
| Guo CM (45) | 2021 | China | Not provided | I–IV | RC | 385 | 57±10 | Univariate | Full range | 2, 3, 4, 5 |
| Chen SL (46) | 2021 | China | G1–G3 | I–IV | RC | 268 | 54.0 | Univariate | Full range | 2 |
| Zang PP (47) | 2020 | China | G1–G3 | Not provided | RC | 84 | 55.3±7.4 | Univariate | Pelvic cavity | 2 |
| Li YJ (48) | 2020 | China | G1–G3 | I–IV | RC | 393 | 56 [25–80] | Univariate | Pelvic cavity and abdominal aorta | 3 |
| Cheng F (49) | 2020 | China | G1–G3 | I–IV | RC | 520 | 55.3±8.4 | Multivariate | Full range | 2 |
| Wang YL (50) | 2019 | China | G1–G3 | Not provided | RC | 192 | Not provided | Multivariate | Full range | 2 |
| Li X (51) | 2019 | China | Not provided | Not provided | RC | 653 | 52.53±8.49 | Multivariate | Full range | 2 |
| Ji R (52) | 2019 | China | Not provided | I–III | RC | 162 | 56.3 | Univariate | Pelvic cavity and abdominal aorta | 2 |
| Liu S (53) | 2018 | China | Not provided | I–III | RC | 176 | 53.74±8.91 | Univariate | Full range | 2 |
| Li Y (54) | 2018 | China | Not provided | Not provided | RC | 1,724 | 55.20±8.72 | Univariate | Full range | 2 |
| Li M (55) | 2018 | China | G1–G3 | Not provided | RC | 74 | 54.32±8.34 | Univariate | Pelvic cavity | 2 |
| Zhang QH (56) | 2017 | China | G1–G3 | I–IV | RC | 136 | 53.46±7.8 | Multivariate | Pelvic cavity | 2 |
| Liang DX (57) | 2017 | China | G1–G3 | Not provided | RC | 210 | 50.12±5.96 | Univariate | Full range | 2 |
| Liu CY (58) | 2017 | China | G1–G3 | I–IV | RC | 366 | 53.734±7.900 | Univariate | Full range | 2 |
| Zeng J (59) | 2017 | China | G1–G3 | I–IV | RC | 289 | 55 [23–78] | Multivariate | Full range | 2 |
| Zhang QH (60) | 2016 | China | G1–G3 | I–IV | RC | 136 | 53.46±7.84 | Multivariate | Full range | 2 |
| Xu Z (61) | 2014 | China | G1–G3 | I–IV | RC | 358 | 50 [20–78] | Univariate | Full range | 2 |
| Yu ML (62) | 2013 | China | G1–G3 | I–IV | RC | 221 | 52.96±8.63 | Multivariate | Full range | 2 |
| Huang J (63) | 2011 | China | G1–G3 | IA–IIIC | RC | 196 | 53.03±8. 9 | Multivariate | Full range | 2 |
| Wang N (64) | 2009 | China | G1–G3 | I–IV | RC | 600 | 54.93±8.36 | Univariate | Full range | 2 |
| Guo XX (65) | 2005 | China | G1–G3 | I–IV | RC | 128 | 55.3 | Univariate | Full range | 2 |
| Cai HB (66) | 2001 | China | G1–G3 | I–II | RC | 156 | 55.2 | Univariate | Full range | 1.5 |
| Khatib G (67) | 2022 | Turkey | G1–G3 | I–IV | RC | 213 | 56 [27–80] | Multivariate | Full range | 2 |
*, data are presented as mean ± SD, median [range], or mean. TD, tumor diameter; LNM, lymph node metastasis; FIGO, International Federation of Gynecology and Obstetrics; RC, retrospective cohort study; SD, standard deviation.
Table 2
| Author | Year | Country/region | Pathological grade | FIGO stage | Type of study | Sample size | Age (years)* | Univariate or multivariate | Cut-off value (cm) |
|---|---|---|---|---|---|---|---|---|---|
| Ocak B (68) | 2021 | Turkey | G1–G3 | I | RC | 284 | 60 [31–81] | Multivariate | Continuous |
| Ocak B (69) | 2021 | Turkey | G1–G3 | I | RC | 272 | 65.0 | Multivariate | Continuous |
| Nwachukwu C (70) | 2021 | USA | G1 | IA | RC | 222 | 59.7±10.6 | Multivariate | 2 |
| Eriksson LSE (71) | 2021 | Sweden | G1–G3 | I–IV | RC | 339 | 67 [60–72] | Multivariate | 2 |
| Liu CY (72) | 2020 | China | G1–G2 | I–III | RC | 238 | 60.0 | Multivariate | 2 |
| Yildirim N (28) | 2018 | Turkey | G1–G4 | I–IV | RC | 278 | 60±9.8 | Univariate | 2 |
| Sozzi G (73) | 2018 | Italy | G1–G3 | I–III | RC | 1,166 | 63.0 | Multivariate | 2.5 |
| Güngördük K (74) | 2018 | Turkey | G1–G2 | IA | RC | 280 | 56.9 | Multivariate | 2 |
| Senol T (19) | 2015 | Turkey | G1–G3 | I–IV | RC | 152 | 56.3 | Univariate | 2 |
| Bendifallah S (75) | 2014 | France | G1–G3 | I–III | RC | 396 | 65.99 [31–86] | Multivariate | 2 |
| Chattopadhyay S (76) | 2013 | England | G1–G3 | I | RC | 216 | 66.0 | Multivariate | Continuous |
| Misirlioglu S (77) | 2012 | Turkey | Not provided | I | RC | 223 | 56 [55–80] | Univariate | 2 |
| Bandyopadhyay S (78) | 2012 | USA | Not provided | I–IV | RC | 123 | 67.2 | Univariate | 2 |
| Guo CM (45) | 2021 | China | Not provided | I–IV | RC | 385 | 57±10 | Univariate | 2, 3, 4, 5 |
| Ma HN (79) | 2020 | China | Not provided | I–II | RC | 257 | 56.4±8.9 | Multivariate | 2 |
| Guo DD (80) | 2020 | China | Not provided | I–II | RC | 702 | 55.0 | Univariate | 2 |
| Tao YZ (81) | 2016 | China | Not provided | I–II | RC | 123 | 55.1±5.2 | Multivariate | 2 |
| Zhong KN (82) | 2015 | China | G1–G3 | I–II | RC | 123 | 54.6±4.9 | Multivariate | 2 |
| Wang L (83) | 2015 | China | G1–G3 | I–II | RC | 120 | 59.5±6.1 | Multivariate | 2 |
| Li MZ (84) | 2014 | China | G1–G3 | I–II | RC | 398 | 57.0 | Univariate | 2 |
| Doll KM (85) | 2014 | USA | G3 | Not provided | RC | 208 | 65.0 | Multivariate | Continuous |
| Shah C (20) | 2005 | USA | G1–G3 | I–IV | RC | 345 | 65.0 | Multivariate | Continuous |
| Zeng J (59) | 2017 | China | G1–G3 | I–IV | RC | 289 | 55 [23–78] | Univariate | 2 |
| Xing XR (86) | 2022 | China | G1–G3 | I–III | RC | 80 | 50.22±5.12 | Univariate | 2 |
| Chen XL (87) | 2022 | China | G1–G3 | I–IV | RC | 94 | 58.24±9.33 | Univariate | 2 |
*, data are presented as mean ± SD, median [range], or mean. TD, tumor diameter; FIGO, International Federation of Gynecology and Obstetrics; RC, retrospective cohort study; SD, standard deviation.
Quality assessment of included studies
All included studies were retrospective and therapeutic research. Quality assessment was conducted for selection, comparability, and outcome/exposure using NOS (a “*” was scored 1 point, and the final score was the sum of all “*”), as shown in Table 3. We included articles with scores of >6 into this study. The higher the quality of the studies included in the meta-analysis, the higher the reliability of the meta-analysis results.
Table 3
| Author | Year | Queue selection | Comparability | Result | Quality score |
|---|---|---|---|---|---|
| Li X | 2021 | **** | * | *** | 8 |
| Meydanli MM | 2019 | *** | * | ** | 6 |
| Matsushita C | 2019 | **** | * | *** | 8 |
| Dong Y | 2019 | **** | * | ** | 7 |
| Nasioudis D | 2019 | *** | * | ** | 6 |
| Günakan E | 2019 | *** | * | *** | 7 |
| Yildirim N | 2018 | **** | * | *** | 8 |
| Toptaş T | 2017 | **** | * | *** | 8 |
| Sari ME | 2017 | **** | * | *** | 8 |
| Lucic N | 2017 | *** | * | ** | 6 |
| Boyraz G | 2017 | ***** | * | ** | 8 |
| Cox Bauer CM | 2016 | ***** | * | ** | 8 |
| Canlorbe G | 2016 | **** | * | ** | 7 |
| Bourgioti C | 2016 | ***** | * | *** | 9 |
| Cetinkaya K | 2016 | *** | * | ** | 6 |
| Bendifallah S | 2015 | **** | * | ** | 7 |
| Rathod PS | 2014 | **** | * | ** | 7 |
| Mahdi H | 2015 | *** | * | ** | 6 |
| Gilani S | 2014 | *** | * | ** | 6 |
| AlHilli MM | 2013 | *** | * | ** | 6 |
| Shah C | 2005 | **** | * | *** | 7 |
| Watanabe M | 2003 | **** | * | ** | 7 |
| Cheng WF | 1998 | *** | * | ** | 6 |
| Wu SW | 2021 | **** | * | ** | 7 |
| Chen SL | 2021 | **** | * | ** | 7 |
| Zang PP | 2020 | **** | * | ** | 7 |
| Li YJ | 2020 | *** | * | *** | 7 |
| Cheng F | 2020 | *** | * | ** | 6 |
| Wang YL | 2019 | *** | * | ** | 6 |
| Li X | 2019 | *** | * | ** | 6 |
| Ji R | 2019 | *** | * | ** | 6 |
| Liu S | 2018 | *** | * | *** | 7 |
| Li Y | 2018 | *** | * | ** | 6 |
| Li M | 2018 | *** | * | ** | 6 |
| Zhang QH | 2017 | *** | * | *** | 7 |
| Liang DX | 2017 | ** | * | *** | 6 |
| Liu CY | 2017 | *** | * | ** | 6 |
| Guo CM | 2021 | *** | * | *** | 7 |
| Xu Z | 2014 | *** | * | *** | 7 |
| Yu ML | 2013 | ** | * | ** | 6 |
| Huang J | 2011 | **** | * | ** | 7 |
| Wang N | 2009 | **** | * | ** | 7 |
| Guo XX | 2005 | ** | * | *** | 6 |
| Cai HB | 2001 | *** | * | ** | 6 |
| Ocak B | 2021 | ***** | * | *** | 8 |
| Ocak B | 2021 | ***** | * | **** | 9 |
| Nwachukwu C | 2021 | *** | * | *** | 7 |
| Eriksson LSE | 2021 | **** | * | ** | 8 |
| Liu CY | 2020 | *** | * | ** | 6 |
| Yildirim N | 2018 | *** | * | ** | 6 |
| Sozzi G | 2018 | **** | * | ** | 6 |
| Güngördük K | 2018 | *** | * | *** | 7 |
| Senol T | 2015 | *** | * | ** | 6 |
| Bendifallah S | 2014 | *** | * | ** | 6 |
| Chattopadhyay S | 2013 | *** | * | ** | 6 |
| Misirlioglu S | 2012 | **** | * | ** | 7 |
| Bandyopadhyay S | 2012 | *** | * | ** | 6 |
| Ma HN | 2020 | **** | * | ** | 7 |
| Guo DD | 2020 | *** | * | ** | 6 |
| Tao YZ | 2016 | *** | * | ** | 6 |
| Zhong KN | 2015 | *** | * | *** | 7 |
| Wang L | 2015 | **** | * | ** | 7 |
| Li MZ | 2014 | *** | * | ** | 6 |
| Doll KM | 2014 | **** | * | ** | 7 |
| Shah C | 2005 | *** | * | ** | 6 |
| Zeng J | 2017 | *** | * | ** | 6 |
| Khatib G | 2022 | ** | ** | ** | 6 |
| Xing XR | 2022 | ** | ** | ** | 6 |
| Chen XL | 2022 | *** | ** | ** | 7 |
A “*” was scored 1 point, and the final score was the sum of all “*”. NOS, Newcastle-Ottawa Scale.
Association of TD with LNM
Results of meta-analysis for the association of TD with LNM
There were 48 studies that reported TD and LNM. Among them, 35 studies used TD =2 cm as the cut-off value. Heterogeneity among the studies was considered (I2=77.5%; P=0.000), and the effects were pooled using random-effect model. The forest plot showed that LNM risk in EC patients with the TD >2 cm was 2.88 times higher than that in those with ≤2 cm (OR =2.88; 95% CI: 2.12–3.89; P<0.001) (Figure 2).
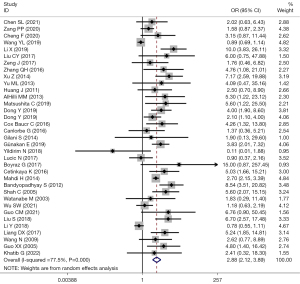
Subgroup analysis
An overview of the factors that might affect the results showed that participant’s or the author’s continents, the manifestation of the study results, and the pathological grades might be the source of heterogeneity. Subgroup analysis was performed based on these factors, and the heterogeneity results were provided. Inclusion of participants’ continents, pathological grades, and FIGO stages yielded various heterogeneity, suggesting that those factors might be the source of heterogeneity (Table 4).
Table 4
| Subgroup category | Number of documents included | OR | 95% CI | P value | I2 | Q test P value |
|---|---|---|---|---|---|---|
| Continents | ||||||
| Asia | 27 | 2.83 | 1.97–4.07 | <0.001 | 0.769 | 0.000 |
| North America | 6 | 4.18 | 2.54–6.89 | 0.124 | 0.422 | 0.124 |
| Europe | 2 | 1.02 | 0.49–2.12 | 0.606 | 0.000 | 0.606 |
| Univariate or multivariate | ||||||
| Univariate | 26 | 2.85 | 2.06–3.95 | <0.001 | 0.729 | 0.000 |
| Multivariate | 9 | 3.02 | 1.35–6.74 | <0.001 | 0.785 | 0.000 |
| Pathological grade | ||||||
| G1–G3 | 25 | 2.67 | 1.90–3.74 | <0.001 | 0.667 | 0.000 |
| G1–G4 | 2 | 0.75 | 0.04–16.10 | 0.026 | 0.799 | 0.026 |
| G1–G2 | 2 | 3.91 | 0.54–28.37 | 0.222 | 0.329 | 0.222 |
| FIGO stage | ||||||
| I–IV | 16 | 4.14 | 3.06–5.62 | <0.001 | 0.006 | 0.445 |
| I–III | 6 | 2.99 | 1.51–5.92 | 0.002 | 0.589 | 0.033 |
| I–II | 7 | 1.68 | 0.96–2.95 | <0.001 | 0.124 | 0.331 |
| IA | 1 | 15 | 0.87–257.44 | 0.062 | – | – |
| I | 1 | 2.7 | 2.15–3.39 | <0.001 | – | – |
TD, tumor diameter; LNM, lymph node metastasis; OR, odds ratio; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics.
The association of different TD cut-off value with LNM
The summary of included studies showed that the selected cut-off value varied among different studies in discussing the influence of TD on LNM (1.5, 2, 2.5, 3, 3.5, and 5 cm, respectively). Subgroups were set based on different cut-off values to explore their association with LNM, as shown in Table 5.
Table 5
| TD cut-off value (cm) | Number of documents included | OR | 95% CI | P value | I2 | Q test P value |
|---|---|---|---|---|---|---|
| 1.5 | 3 | 1.14 | 0.43–3.00 | 0.796 | 0.0 | 0.744 |
| 2 | 35 | 2.88 | 2.12–3.89 | <0.001 | 0.782 | 0.000 |
| 2.5 | 1 | 12.571 | 1.437–110.009 | 0.022 | – | – |
| 3 | 3 | 3.27 | 1.91–5.79 | <0.001 | 0.208 | 0.283 |
| 3.5 | 1 | 4.318 | 1.129–16.511 | 0.033 | – | – |
| 4 | 4 | 3.6 | 2.44–5.31 | <0.001 | 0.375 | 0.187 |
| 5 | 3 | 3.46 | 1.81–6.61 | <0.001 | 0.832 | 0.003 |
TD, tumor diameter; LNM, lymph node metastasis; OR, odds ratio; CI, confidence interval.
Publication bias and sensitivity analysis
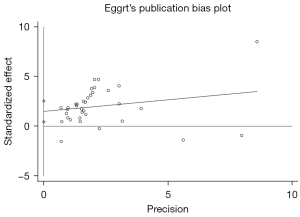
Egger’s test was adopted to assess the publication bias, and the results showed no publication bias (P=0.07), which means our results are highly reliable, as shown in Figure 3. After removal of any of the studies, the pooled effects of the rest of the studies were in the 95% CI range of the total effect, which suggested that the results were robust (Figure 4).

Association of TD with recurrence
Results of meta-analysis for the association of TD with recurrence
There were 25 studies that reported the association between TD and EC recurrence. Among them, there 18 studies used TD =2 cm as the cut-off value. Significant heterogeneity was considered among the studies (I2=89.3%; P=0.000), and the effects were pooled using random-effect model. The recurrence risk in EC patients with TD >2 cm was 2.45 times higher than that in those with ≤2 cm (OR =2.45; 95% CI: 1.73–3.48; P<0.001) (Figure 5).
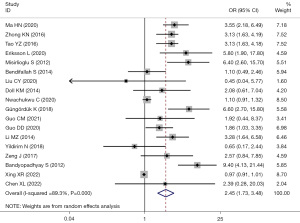
Subgroup analysis
A summary of the factors that might affect the results showed that participants’ or the author’s continents, the manifestation of the study results, and the pathological grades might be the source of heterogeneity. Subgroup analysis was performed based on these factors, and the heterogeneity results were provided. Inclusion of participants’ continents, pathological grades, and FIGO stages yielded various heterogeneity, suggesting that those factors might be the source of heterogeneity (Table 6).
Table 6
| Subgroup category | Number of documents included | OR | 95% CI | P value | I2 | Q test P value |
|---|---|---|---|---|---|---|
| Continents | ||||||
| Asia | 13 | 2.44 | 1.45–4.10 | 0.001 | 0.090 | 0.000 |
| North America | 3 | 2.72 | 0.62–11.82 | 0.183 | 0.92 | 0.000 |
| Europe | 2 | 2.41 | 0.47–12.26 | 0.289 | 0.821 | 0.018 |
| Univariate or multivariate | ||||||
| Univariate | 9 | 2.44 | 1.24–4.79 | 0.001 | 0.879 | 0.000 |
| Multivariate | 9 | 2.49 | 1.45–4.25 | 0.010 | 0.858 | 0.000 |
| Pathological grade | ||||||
| G1–G3 | 6 | 2.53 | 1.18–5.44 | <0.001 | 0.896 | 0.000 |
| G1–G4 | 1 | 0.65 | 0.17–2.44 | 0.520 | – | – |
| G1–G2 | 1 | 6.6 | 2.70–15.80 | <0.001 | – | – |
| G1 | 1 | 1.1 | 0.91–1.32 | 0.351 | – | – |
| G3 | 1 | 2.08 | 0.61–7.07 | 0.241 | – | – |
| FIGO stage | ||||||
| I–IV | 6 | 3.01 | 1.31–6.94 | 0.010 | 0.629 | 0.019 |
| I–III | 3 | 0.97 | 0.92–1.02 | 0.217 | 0.00 | 0.794 |
| I–II | 5 | 2.96 | 2.33–3.76 | <0.001 | <0.001 | 0.554 |
| IA | 2 | 2.55 | 0.44–14.73 | 0.295 | 0.934 | 0.000 |
| I | 1 | 6.4 | 2.60–15.70 | <0.001 | – | – |
TD, tumor diameter; OR, odds ratio; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics.
Association of different TD cut-off value with EC recurrence
The summary of included studies showed that the selected cut-off value varied among different studies in discussing the influence of TD on EC recurrence (2, 2.5, and 3.75 cm, respectively). Subgroup analysis was performed and the results are shown in Table 7.
Table 7
| TD cut-off value (cm) | Number of documents included | OR | 95% CI | P value | I2 | Q test P value |
|---|---|---|---|---|---|---|
| 2 | 18 | 2.45 | 1.73–3.48 | <0.001 | 0.893 | 0.000 |
| 2.5 | 1 | 18.7 | 2.4–140.3 | <0.001 | – | – |
| 3.75 | 1 | 7.9 | 2.2–28.9 | 0.031 | – | – |
TD, tumor diameter; OR, odds ratio; CI, confidence interval.
Publication bias and sensitivity analysis
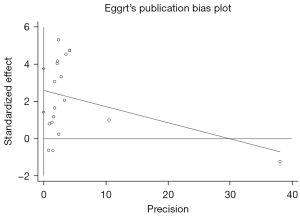
Egger’s test was adopted to assess the publication bias, and the result indicated the presence of significant publication bias (P=0.000), which means that our results are heavily influenced by publication bias and more research is needed, as shown in Figure 6. After removal of any of the studies, the pooled effects of the rest of the studies were in the 95% CI range of the total effect (Figures 2,3), which suggested that the results were robust (Figure 7).
Discussion
In this study, we extracted the data of included studies, and found that most of the studies followed the Mayo standard and the National Comprehensive Cancer Network (NCCN) guidelines. Both criteria considered TD less than 2 cm as a low risk factor for LNM in EC. We selected 2 cm as the cut-off value of TD. Participants with the TD <2 cm were assigned into LNM low-risk group. Yildirim et al. (28) conducted a study that involved 278 patients at I–IV stage. They found that TD was unassociated with LNM, and the positive rate of LN was 3/46 (6.5%) in EC patients with a TD <2 cm, and 10/232 (4.3%) in those with a TD ≥2 cm (P=0.457). LVSI and positive ascites cytology were considered crucial risk factors. Their findings were inconsistent with our study, which might be caused by bias due to its retrospective-design. Additionally, LNM or recurrence had happened in few of the participants leading to too limited a sample size to perform the most robust statistical inference. An internal and external validation study by Dong et al. (25), constructed a nomogram based on 700 EC patients from Peking University People’s Hospital, and validated the information of 727 EC patients from the cancer center of Fudan University. They found that in both of the populations, the LNM risk in EC patients with the TD ≥2 cm was 2.1 and 4.0 times higher, respectively, than that in those with the TD <2 cm [(OR =2.1; 95% CI: 1.1–4.0; P=0.019), (OR =4.0; 95% CI: 1.9–8.6; P≤0.001), respectively].
In this study, 35 articles with a TD cut-off value of 2 cm were included and analyzed. The results showed that LNM risk in EC patients with the TD >2 cm was 2.88 times higher than that in those with the TD ≤2 cm, and the difference was statistically significant (OR =2.88; 95% CI: 2.12–3.89; P<0.001). Heterogeneity showed an I2=77.5%, and Q test showed that P=0.000. Further heterogeneity analysis was performed due to the existing significant heterogeneity. We found that the origin of the participants, FIGO stages, and pathological grades affected the results. This also suggested that there was a certain association of TD with EC stages and pathological grades. Publication bias assessment showed that publication bias exerted no influence on the results. Therefore, the high risk of LNM should be considered for EC patients with the TD >2 cm in clinical practice. TD is an important staging criterion for lung cancer and breast cancer, yet the mechanism of TD in EC staging and treatment remains elusive, which has been confirmed by our study.
LNM in EC patients represents a jumping process, which is different from the stepped process in cervical cancer patients. Even if there is no evidence of metastasis in PLN, cancer cells might migrate to PALN through the infundibulopelvic ligament. Therefore, some researchers have studied the association of TD with PLN and PALN, respectively. Five of included studies were divided into PLNM and para-aortic LNM (PALNM) according to the association of TD with LNM and the metastatic site. The risk of PLNM increased by 4.71 times in patients with the TD >2 cm (OR =4.71; 95% CI: 0.04–15.10; P=0.000), and the risk of PALN in patients with the TD >2 cm was 3.97 times higher than that in those with ≤2 cm (OR =3.97; 95% CI: 1.46–10.79; P=0.007). Stimulation was conducted using random-effect model and fixed-effect model, and the results were stable, which was in consistence with the results of studies mentioned above.
As a prognostic factor, TD is always associated with LNM, whereas the association of TD with EC recurrence is unclear (36,88,89). A study by Çakır et al. (90) revealed a 5-year DFS of 94% in EC patients with the TD <3.5 cm, and 89% in those with the TD >3.5 cm (P=0.128). TD failed to be a risk factor for post-LND recurrence in EC patients. Among the 17 studies that were finally included, most applied a TD cut-off value of 2 cm to assess the risk of recurrence. The results showed that the risk of recurrence in EC patients with the TD >2 cm was 2.45 times higher than that in those with the TD ≤2 cm (OR =2.45; 95% CI: 1.73–3.48; P<0.001). Significant heterogeneity existed among the studies (I2=89.3%; P=0.000). The source of heterogeneity might be participants’ continents, pathological grades, and FIGO stages. Publication bias assessment showed that publication bias exerted influence on the results. More RCT studies are needed to confirm the relationship between TD and recurrence It is worth noting that some studies have proposed that the risk of recurrence rise follows the increase of TD in EC patients, and the difference was statistically significant (76,83). There are also some studies (20,68,85) which have presented the opposite attitude. This situation may be related to the FIGO stage and pathological grade of the participants. For example, study by Ocak et al. (68,69) recruited only FIGO stage-I patients, and the risk of early recurrence in these patients would be relatively low, so that it could not provide the best conclusion. If all the patients included had high pathological grade, the contribution of TD to recurrence might be masked due to the tendency of local and distant recurrence of highly malignant diseases (85).
Limitations
Our study also had some limitations. All extracted data were from published articles, and only part of the data contained patients’ original information. All included studies were retrospectively designed so that the strength of evidence was lower that the evidence produced by prospective randomized controlled trials. There were few studies focusing on the association of TD with EC recurrence leading to bias in the results. The inclusion and exclusion criteria varied among the studies leading to various dependent variables, which might induce bias in the results, even though the potential source of heterogeneity was analyzed. The lack of uniform standard for TD measurement might have affected the TD. TD measurement on hysterectomy specimens did not consider the effect of preoperative biopsy on TD.
Conclusions
EC patients with a TD >2 cm have a higher risk of LNM than those with a TD ≤2 cm. The risk of LNM and recurrence rises alongside the increase of TD in EC patients.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2595/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2595/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kitchener HC, Trimble ELEndometrial Cancer Working Group of the Gynecologic Cancer Intergroup. Endometrial cancer state of the science meeting. Int J Gynecol Cancer 2009;19:134-40. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Dinkelspiel HE, Wright JD, Lewin SN, et al. Contemporary clinical management of endometrial cancer. Obstet Gynecol Int 2013;2013:583891. [Crossref] [PubMed]
- Obermair A, Youlden DR, Young JP, et al. Risk of endometrial cancer for women diagnosed with HNPCC-related colorectal carcinoma. Int J Cancer 2010;127:2678-84. [Crossref] [PubMed]
- Seebacher V, Schmid M, Polterauer S, et al. The presence of postmenopausal bleeding as prognostic parameter in patients with endometrial cancer: a retrospective multi-center study. BMC Cancer 2009;9:460. [Crossref] [PubMed]
- Barnes MN, Kilgore LC. Complete surgical staging of early endometrial adenocarcinoma: optimizing patient outcomes. Semin Radiat Oncol 2000;10:3-7. [Crossref] [PubMed]
- Sari ME, Yalcin İ, Sahin H, et al. Risk factors for paraaortic lymph node metastasis in endometrial cancer. Int J Clin Oncol 2017;22:937-44. [Crossref] [PubMed]
- Garg G, Morris RT, Solomon L, et al. Evaluating the significance of location of lymph node metastasis and extranodal disease in women with stage IIIC endometrial cancer. Gynecol Oncol 2011;123:208-13. [Crossref] [PubMed]
- Morrow CP, Bundy BN, Kurman RJ, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol 1991;40:55-65. [Crossref] [PubMed]
- Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987;60:2035-41. [Crossref] [PubMed]
- Bougherara L, Azaïs H, Béhal H, et al. Does lymphadenectomy improve survival in patients with intermediate risk endometrial cancer? A multicentric study from the FRANCOGYN Research Group. Int J Gynecol Cancer 2019;29:282-9. [Crossref] [PubMed]
- Karalok A, Turan T, Basaran D, et al. Lymph Node Metastasis in Patients With Endometrioid Endometrial Cancer: Overtreatment Is the Main Issue. Int J Gynecol Cancer 2017;27:748-53. [Crossref] [PubMed]
- Vargas R, Rauh-Hain JA, Clemmer J, et al. Tumor size, depth of invasion, and histologic grade as prognostic factors of lymph node involvement in endometrial cancer: a SEER analysis. Gynecol Oncol 2014;133:216-20. [Crossref] [PubMed]
- Colombo N, Preti E, Landoni F, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi33-8. [Crossref] [PubMed]
- Bendifallah S, Canlorbe G, Raimond E, et al. A clue towards improving the European Society of Medical Oncology risk group classification in apparent early stage endometrial cancer? Impact of lymphovascular space invasion. Br J Cancer 2014;110:2640-6. [Crossref] [PubMed]
- Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2004;92:744-51. [Crossref] [PubMed]
- Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol 2016;27:16-41. Erratum in: Ann Oncol 2017;28:iv167-8. [Crossref] [PubMed]
- Schink JC, Rademaker AW, Miller DS, et al. Tumor size in endometrial cancer. Cancer 1991;67:2791-4. [Crossref] [PubMed]
- Senol T, Polat M, Ozkaya E, et al. Tumor Diameter for Prediction of Recurrence, Disease Free and Overall Survival in Endometrial Cancer Cases. Asian Pac J Cancer Prev 2015;16:7463-6. [Crossref] [PubMed]
- Shah C, Johnson EB, Everett E, et al. Does size matter? Tumor size and morphology as predictors of nodal status and recurrence in endometrial cancer. Gynecol Oncol 2005;99:564-70. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Li X, Cheng Y, Dong Y, et al. Development and validation of predictive model for lymph node metastasis in endometrial cancer: a SEER analysis. Ann Transl Med 2021;9:538. [Crossref] [PubMed]
- Meydanli MM, Aslan K, Oz M, et al. A novel multivariable prediction model for lymphatic dissemination in endometrioid endometrial cancer: The lymph node Metastasis Risk Index. Eur J Obstet Gynecol Reprod Biol 2019;240:310-5. [Crossref] [PubMed]
- Matsushita C, Fujiwara H, Takei Y, et al. New criteria for the omission of lymphadenectomy in endometrioid carcinoma. Int J Gynecol Cancer 2019;29:541-6. [Crossref] [PubMed]
- Dong Y, Cheng Y, Tian W, et al. An Externally Validated Nomogram for Predicting Lymph Node Metastasis of Presumed Stage I and II Endometrial Cancer. Front Oncol 2019;9:1218. [Crossref] [PubMed]
- Nasioudis D, Holcomb K. Incidence of isolated para-aortic lymph node metastasis in early stage endometrial cancer. Eur J Obstet Gynecol Reprod Biol 2019;242:43-6. [Crossref] [PubMed]
- Günakan E, Atan S, Haberal AN, et al. A novel prediction method for lymph node involvement in endometrial cancer: machine learning. Int J Gynecol Cancer 2019;29:320-4. [Crossref] [PubMed]
- Yildirim N, Bilgi A, Gokulu SG, et al. Lymphovascular space invasion and positive peritoneal cytology are independent prognostic factors for lymph node metastasis and recurrence in endometrial cancer. Eur J Gynaecol Oncol 2018;39:977-83.
- Toptaş T, Şimşek T, Karaveli Ş. Prognostic risk factors for lymph node involvement in patients with endometrial cancer. Turk J Obstet Gynecol 2017;14:52-7. [Crossref] [PubMed]
- Lucic N, Draganovic D, Sibincic S, et al. Myometrium Invasion, Tumour Size and Lymphovascular Invasion as a Prognostic Factor in Dissemination of Pelvic Lymphatics at Endometrial Carcinoma. Med Arch 2017;71:325-9. [Crossref] [PubMed]
- Boyraz G, Salman MC, Gultekin M, et al. Incidence of Lymph Node Metastasis in Surgically Staged FIGO IA G1/G2 Endometrial Cancer With a Tumor Size of More Than 2 cm. Int J Gynecol Cancer 2017;27:486-92. [Crossref] [PubMed]
- Cox Bauer CM, Greer DM, Kram JJF, et al. Tumor diameter as a predictor of lymphatic dissemination in endometrioid endometrial cancer. Gynecol Oncol 2016;141:199-205. [Crossref] [PubMed]
- Canlorbe G, Bendifallah S, Laas E, et al. Tumor Size, an Additional Prognostic Factor to Include in Low-Risk Endometrial Cancer: Results of a French Multicenter Study. Ann Surg Oncol 2016;23:171-7. [Crossref] [PubMed]
- Bourgioti C, Chatoupis K, Tzavara C, et al. Predictive ability of maximal tumor diameter on MRI for high-risk endometrial cancer. Abdom Radiol (NY) 2016;41:2484-95. [Crossref] [PubMed]
- Cetinkaya K, Atalay F, Bacinoglu A, et al. To what extent is risk grouping method successful in deciding surgical staging in endometrial cancer? Tumori 2016;102:422-5. [Crossref] [PubMed]
- Bendifallah S, Canlorbe G, Arsène E, et al. French Multicenter Study Evaluating the Risk of Lymph Node Metastases in Early-Stage Endometrial Cancer: Contribution of a Risk Scoring System. Ann Surg Oncol 2015;22:2722-8. [Crossref] [PubMed]
- Bendifallah S, Canlorbe G, Laas E, et al. A Predictive Model Using Histopathologic Characteristics of Early-Stage Type 1 Endometrial Cancer to Identify Patients at High Risk for Lymph Node Metastasis. Ann Surg Oncol 2015;22:4224-32. [Crossref] [PubMed]
- Rathod PS, Shakuntala PN, Pallavi VR, et al. The risk and pattern of pelvic and para aortic lymph nodal metastasis in patients with intermediate and high risk endometrial cancer. Indian J Surg Oncol 2014;5:109-14. [Crossref] [PubMed]
- Mahdi H, Munkarah AR, Ali-Fehmi R, et al. Tumor size is an independent predictor of lymph node metastasis and survival in early stage endometrioid endometrial cancer. Arch Gynecol Obstet 2015;292:183-90. [Crossref] [PubMed]
- Gilani S, Anderson I, Fathallah L, et al. Factors predicting nodal metastasis in endometrial cancer. Arch Gynecol Obstet 2014;290:1187-93. [Crossref] [PubMed]
- AlHilli MM, Podratz KC, Dowdy SC, et al. Risk-scoring system for the individualized prediction of lymphatic dissemination in patients with endometrioid endometrial cancer. Gynecol Oncol 2013;131:103-8. [Crossref] [PubMed]
- Watanabe M, Aoki Y, Kase H, et al. Low risk endometrial cancer: a study of pelvic lymph node metastasis. Int J Gynecol Cancer 2003;13:38-41. [Crossref] [PubMed]
- Cheng WF, Chen CA, Lee CN, et al. Preoperative ultrasound study in predicting lymph node metastasis for endometrial cancer patients. Gynecol Oncol 1998;71:424-7. [Crossref] [PubMed]
- Wu SW, Xie YQ, Feng YF, et al. Correlation Analysis of Lymph Node Metastasis and Prognostic Factors of I-III Stage Endometrial Carcinoma. Journal of Practical Obstetrics and Gynecology 2021;37:689-93.
- Guo CM, Dai YB, Geng J, et al. Correlation between the primary tumor size of endometrial carcinoma and lymph node metastasis and recurrence. Zhonghua Fu Chan Ke Za Zhi 2021;56:264-70. [PubMed]
- Chen SL, Song KR, Liu Y, et al. High-risk factors of lymph node metastasis of endometrial cancer and the construction of prediction model. China Medical Herald 2021;18:31-5.
- Zang PP, Yang ZL. Correlation of contrast enhanced ultrasound parameters and pelvic lymph node metastasis of endometrial carcinoma. Chinese Journal of Medical Imaging Technology 2020;36:1867-71.
- Li YJ. Risk factors and clinical application value of lymph node metastasis in endometrial cancer. Qingdao: Qingdao University, 2020.
- Cheng F, Chen X, Fang CY, et al. Risk factors of lymph node metastasis in endometrial carcinoma. Zhejiang Medical Journal 2020;42:2529-32.
- Wang YL, Ma HS, Li YJ. Study on lymph Node Metastasis and Predictive Model of endometrial Cancer. Inner Mongolia Medical Journal 2019;51:1469-71.
- Li X. Analysis of lymph node metastasis and risk factors of type I endometrial carcinoma. Hengyang: Nanhua University, 2019.
- Ji R, Lu YY. The correlation analysis between clinical pathological characteristics and pelvic or para-aortic lymph node metastasis in endometrial carcinoma. Journal of Modern Oncology 2019;27:3491-4.
- Liu S, Xu XY, Hu JJ, et al. Analysis on related factors of lymph node metastasis in endometrial cancer. Xinjiang Medical Journal 2018;48:984-7.
- Li Y. Analysis of lymph node metastasis and high risk factors in endometrial carcinoma. Qingdao: Qingdao University, 2018.
- Li M. Relationship between pathological features and pelvic lymph node metastasis in patients with endometrial carcinoma. Women's Health Research 2018;22:74, 149.
- Zhang QH, Xu WS, Lan X. Analysis on Related Factors of Pelvic Lymph Node Metastasis in Patients with Type I Endometrial Carcinoma. Journal of Practical Obstetrics and Gynecology 2017;33:603-5.
- Liang DX, Qiu HJ, Ji YQ. The relationship of clinical and pathological characteristics and lymph node metastasis in patients with endometrial cancer. Journal of Clinical and Experimental Medicine 2017;16:1816-8.
- Liu CY. Risk assessment of lymph node metastasis and lymphangiogenesis in endometrial carcinoma. Tianjin: Tianjin Medical University, 2017.
- Zeng J, Li Y, Jin Y, et al. High-risk factors and prognostic analysis of pelvic nodal metastasis in patients with endometrial carcinoma. Basic & Clinical Medicine 2017;37:454-62.
- Zhang QH. Analysis of related factors of pelvic lymph node metastasis in patients with type I endometrial carcinoma. Nanning: Guangxi Medical University, 2016.
- Xu Z, Peng ZL, Zeng LQ, et al. Clinicopathologic Features and Risk Factors for Pelvic Lymph Node Metastasis in Uterine Endometrioid Cancer: 358 Cases Report. J Chinese Journal of Clinical Medicine of Women and Children 2014;10:37-41. (Electronic Edition).
- Yu ML. Analysis of influencing factors of pelvic lymph node metastasis in endometrial carcinoma. Changchun: Jilin University, 2013.
- Huang J, Gu MJ, Su YY. etal. Use preoperative diffusion weighted MRI imaging and serum CA125 level to predict lymph node metastasis in endometrial cancer. Chinese Journal of Practical Gynecology and Obstetrics 2011;27:676-9.
- Wang N. Clinical analysis of 600 cases of endometrial carcinoma. Shijiazhuang: Hebei Medical University, 2009.
- Guo XX, Yang RX, Zou BY, et al. Clinical value of surgical-pathologic staging for endometrial carcinoma. Practical Journal of Clinical Medicne 2005;2:35-7.
- Cai HB. Prognostic factors affecting lymph node metastasis of endometrial carcinoma. Tumor 2001;21:394.
- Khatib G, Vardar MA, Güzel AB, et al. Predictability of lymph node involvement in uterus-confined endometrioid endometrial cancer by tumour size, pattern and location measured with transvaginal ultrasonography: can we save time? J Obstet Gynaecol 2022; Epub ahead of print. [Crossref] [PubMed]
- Ocak B, Sahin AB, Oz Atalay F, et al. Why do some patients with stage 1A and 1B endometrial endometrioid carcinoma experience recurrence? A retrospective study in search of prognostic factors. Ginekol Pol 2021; Epub ahead of print. [Crossref] [PubMed]
- Ocak B, Atalay FÖ, Sahin AB, et al. The impact of Ki-67 index, squamous differentiation, and several clinicopathologic parameters on the recurrence of low and intermediate-risk endometrial cancer. Bosn J Basic Med Sci 2021;21:549-54. [Crossref] [PubMed]
- Nwachukwu C, Baskovic M, Von Eyben R, et al. Recurrence risk factors in stage IA grade 1 endometrial cancer. J Gynecol Oncol 2021;32:e22. [Crossref] [PubMed]
- Eriksson LSE, Nastic D, Lindqvist PG, et al. Sonographic, demographic characteristics, and the Proactive Molecular Risk Classifier for Endometrial cancer (ProMisE) in the prediction of tumor recurrence or progression. Ultrasound in Obstetrics & Gynecology 2021;58:457-68. [Crossref] [PubMed]
- Liu C, Zhao J, Liu S, et al. Effect of Pelvic Lymphadenectomy on Survival in Patients with Low-Risk Early-Stage Endometrial Cancer Diagnosed Intraoperatively Using Frozen Tissue Sections: A Retrospective Analysis. Cancer Manag Res 2020;12:10715-23. [Crossref] [PubMed]
- Sozzi G, Uccella S, Berretta R, et al. Tumor Size, an Additional Risk Factor of Local Recurrence in Low-Risk Endometrial Cancer: A Large Multicentric Retrospective Study. Int J Gynecol Cancer 2018;28:684-91. [Crossref] [PubMed]
- Güngördük K, Firat Cüylan Z, Kahramanoglu I, et al. Risk Factors for Recurrence in Low-Risk Endometrial Cancer: A Case-Control Study. Oncol Res Treat 2018;41:466-70. [Crossref] [PubMed]
- Bendifallah S, Canlorbe G, Huguet F, et al. A risk scoring system to determine recurrence in early-stage type 1 endometrial cancer: a French multicentre study. Ann Surg Oncol 2014;21:4239-45. [Crossref] [PubMed]
- Chattopadhyay S, Cross P, Nayar A, et al. Tumor size: a better independent predictor of distant failure and death than depth of myometrial invasion in International Federation of Gynecology and Obstetrics stage I endometrioid endometrial cancer. Int J Gynecol Cancer 2013;23:690-7. [Crossref] [PubMed]
- Misirlioglu S, Guzel AB, Gulec UK, et al. Prognostic factors determining recurrence in early-stage endometrial cancer. Eur J Gynaecol Oncol 2012;33:610-4. [PubMed]
- Bandyopadhyay S, Van de Vijver KK, Oliva E, et al. Tumor Size as a Prognostic Factor in Uterine Serous Carcinoma: A Large Multi-Institutional Study. In: Laboratory Investigation. New York: Nature Publishing Group, 2012;92:259A.
- Ma HN, Li YB, Huo JN, et al. Recurrence-Related Factors of 257 Patients with Stage I-II Endometrial Carcinoma. Journal of Cancer Control and Treatment 2020;33:428-32.
- Guo DD. Analysis of relapse and related therapeutic factors in 702 cases of early endometrial carcinoma (stage I~II). Shijiazhuang: Hebei Medical University, 2020.
- Tao YZ, Ding CY, Yue Y. Analysis of clinicopathological factors affecting recurrence and prognosis of patients with stage I-II endometrial carcinoma. Zhejiang Clinical Medical Journal 2016;18:1421-2.
- Zhong KN, Su YY, Han YH, et al. Analysis of clinicopathological factors affecting the recurrence and prognosis of stage I-II endometrial cancer. Chinese Journal of Modern Drug Application 2015;9:62-3.
- Wang L, Yang N. Related Factors Affecting Recurrence and Prognosis of Stage I and II Endometrial Carcinoma. The Practical Journal of Cancer 2015;10:1546-9.
- Li M, Wang Z. Predictors of recurrence and prognosis in patients with stage I and II endometrial carcinoma. Zhonghua Fu Chan Ke Za Zhi 2014;49:455-9. [PubMed]
- Doll KM, Tseng J, Denslow SA, et al. High-grade endometrial cancer: revisiting the impact of tumor size and location on outcomes. Gynecol Oncol 2014;132:44-9. [Crossref] [PubMed]
- Xing XR. Influencing factors of postoperative recurrence of type I endometrial carcinoma. Medical Journal of Chinese People’s Health 2022;34:12-5.
- Chen XL, Zhang WP. Correlation between size of primary lesion and metastasis and recurrence of endometrial cancer. Maternal and Child Health Care of China 2022;37:409-11.
- Kang S, Nam JH, Bae DS, et al. Preoperative assessment of lymph node metastasis in endometrial cancer: A Korean Gynecologic Oncology Group study. Cancer 2017;123:263-72. [Crossref] [PubMed]
- Koskas M, Bassot K, Graesslin O, et al. Impact of lymphovascular space invasion on a nomogram for predicting lymph node metastasis in endometrial cancer. Gynecol Oncol 2013;129:292-7. [Crossref] [PubMed]
- Çakır C, Kılıç İÇ, Yüksel D, et al. Does tumor size have prognostic value in patients undergoing lymphadenectomy in endometrioid-type endometrial cancer confined to the uterine corpus? Turk J Med Sci 2019;49:1403-10. [Crossref] [PubMed]
(English Language Editor: J. Jones)




