
Bartholin’s gland carcinoma—the diagnostic and management challenges of a rare malignancy—a case report and review of current literature
Introduction
Bartholin’s gland carcinoma (BGC) is uncommon. Typically presenting at a more advanced stage, this tumour type is associated with higher rates of recurrence and poorer prognosis than other vulval cancers. Attributed to both rarity and overlap in clinical presentation with benign pathologies of the Bartholin’s gland, mis-diagnosis and subsequent delays in treatment frequently occur. Hence, sharing experience of primary BGC serves to generate greater clinician awareness and highlight the need for diagnostic vigilance. With no current dedicated guidance to aid the gynaecological-oncologist, management remains a challenge and largely based on both the extrapolation of approach to other, better understood vulval cancer sub-types and outcomes from small, retrospective studies. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-612/rc).
Case presentation
A 64-year-old lady was referred to the gynaecological-oncology clinic via the red flag pathway with a new vaginal swelling. The patient was otherwise asymptomatic—denying any associated pain, discharge or bleeding. She had a normal smear history; no significant past gynae or past medical history; had undergone no previous surgery; and was taking no regular medications. The patient was a non-smoker, with normal body mass index (BMI).
Inspection of the external genitalia was unremarkable. Vaginal examination, however, revealed a 3×4 centimetre (cm) swelling in the anatomical position of the right Bartholin’s gland. The mass was fixed and non-tender; extending postero-laterally into the ischio-rectal fossa, without breaching the vaginal or rectal mucosa. There were no clinically palpable groin nodes. The differential diagnosis included a Bartholin’s gland cancer, abscess or cyst. The clinical characteristics of the tumour on examination, however, favoured an underlying malignant pathology.
An MRI pelvis was undertaken—reporting a predominantly solid 3.3 cm × 2.3 cm mass involving both the vulva and postero-lateral aspect of the vaginal introitus. The tumour was described as extending laterally to abut the right levator muscle; and posteriorly to abut, but not obviously invade, the right antero-lateral wall of the anus. A size significant (11 millimetre) right inguinal node was also identified (Figures 1,2). Staging CT-thorax/abdomen/pelvis (CT-TAP) did not identify any additional lymphadenopathy, nor evidence of distant metastases. CT-positron-emission tomography (PET) was also undertaken, confirming a markedly F-fluorodeoxyglucose (FDG)-avid 3 cm right Bartholin’s gland tumour, and moderately FDG-avid right inguinal lymph node (Figures 3,4).
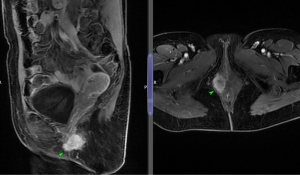
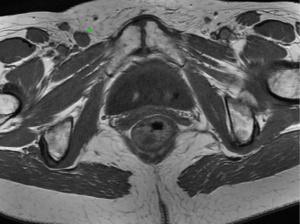
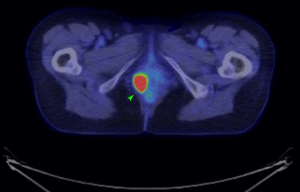
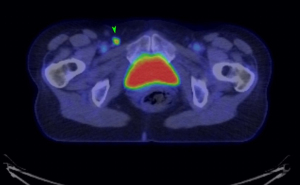
The case was discussed and images reviewed via the gynaecological-oncology multi-disciplinary team (MDT) pathway, with input from gynaecological-oncology surgeons and radiology—and conclusion reached that the clinical picture, together with appearance of tumour on multiple imaging modalities, was in keeping with stage III primary BGC. The MDT recommendation to offer primary surgical management was made.
The patient was reviewed again pre-operatively by a consultant gynaecological-oncologist with cancer nurse specialist (CNS) support. The findings on imaging and MDT recommendation for management were relayed. Extensive pre-operative counselling provided clear explanation of the risks associated with the proposed surgery. Dedicated time and careful attention were given to ensuring patient understanding of the potential need for flap reconstruction or bowel resection with stoma formation as additional procedures dependent on the extent of excision required to achieve clear surgical margins. The future impact of surgery on bowel/bladder and sexual function—as well as the risk of lymphoedema—were also discussed at length. The patient was re-examined, with findings in keeping with those at initial presentation—confirming no overt progression of the tumour, remaining grossly clear of the urethra, bladder, rectum or anal sphincters. Following the above discussions, the patient—concerned regarding the physical and psychological burden of concurrent vulval and groin surgeries—opted to undergo two-stage primary surgery, favouring a step-wise approach in light of still processing the new diagnosis.
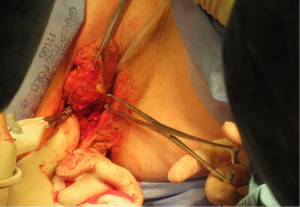
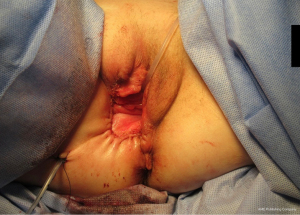
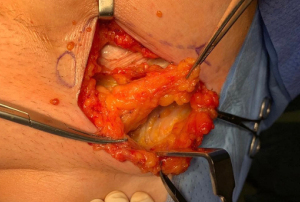
Radical local excision of the Bartholin’s tumour was undertaken. Intra-operative antibiotic prophylaxis was administered and patient catheterised. An examination under anaesthetic (EUA) was performed and a 2 cm fresh margin marked which spared both the urethra and anal verge. An advanced energy device was used to facilitate haemostatic dissection and excision from the tumour bed, carefully guarding the anal sphincters and rectum at the inferior and posterior borders (Figure 5). Tension-free primary wound closure was possible without the need for flap reconstruction. Deeper subcutaneous layers were closed using interrupted 2-0 polysorb (dyed) sutures; and a Redivac drain placed and secured. Skin edges were apposed with 2-0 polysorb (undyed) sutures in a mattress configuration (Figure 6). The estimated blood loss (EBL) was <50 mLs; and operating time 120 minutes. There were no immediate intra-operative complications. The urinary catheter and wound drain were removed on day 2; the patient given standard venous thrombo-embolism (VTE) prophylaxis; and discharged home well on day 4. No post-operative complications occurred.
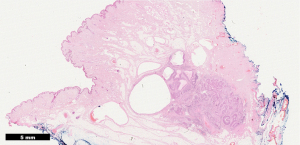
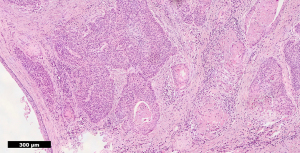
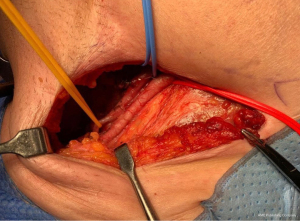
Histopathology confirmed a moderately differentiated squamous cell carcinoma (SCC) of the Bartholin’s gland, with focal lympho-vascular space invasion (LVSI) and positive deep margin (Figures 7,8). The patient was examined at clinic to ensure satisfactory wound healing; before subsequently undergoing completion bilateral groin node dissection (GND) 19 days after primary surgery. Systematic dissection of the right and left inguino-femoral lymph nodes was performed using an advanced energy device and bipolar scissors, preserving the saphenous vein bilaterally (Figures 9,10). A bulky right inguinal node was noted intra-operatively, correlating with pre-operative imaging. Subcutaneous tissue layers were closed using interrupted 2-0 polysorb sutures after bilateral Redivac drain placement (Figure 11); and a subcuticular technique used for skin. The EBL was <50 mLs; and operating time 150 min. No immediate intra-operative complications occurred. The urinary catheter was removed on day 1; and the patient once again given standard VTE prophylaxis. She was discharged home well on day 2, with groin drains in situ. Drain output was monitored as an outpatient; and both removed on day 31. No post-operative complications occurred.
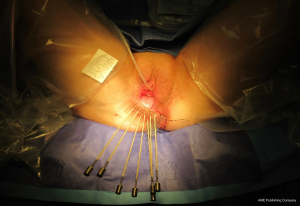
Final histopathology confirmed stage IIIC disease, denoted by two positive right inguinal nodes with extra-capsular spread. The patient was subsequently referred onwards to clinical oncology. She was commenced on Cisplatin chemotherapy 71 days after primary surgery; followed by interstitial brachytherapy to the right paravaginal space (Figure 12).
To date, the patient remains under review with no recurrence 30 months following surgery; nor reporting any long-term disturbance of bladder or bowel function, or lymphoedema.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Epidemiology, demographics and risk factors
United Kingdom (UK) statistics report only 1,300 new cases of vulval carcinoma per year, affecting 1/232 women in their lifetime (1). Carcinoma of the Bartholin’s gland accounts for 2–7.7% of all vulval cancer cases, making it an extremely rare malignancy of the female genital tract (2-5).
Although BGC more commonly occurs in post-menopausal women, the mean age at diagnosis is 57 years–up to 6 years earlier than that for development of a non-BGC of the vulva (2,3,6). Rare cases of BGC in females as young as 29 years have, however, been described (7).
Asides from increasing age, other risk factors for BGC are not entirely clear (8). One study of 33 patients reported a significantly higher incidence amongst African Americans; but did not identify any association with smoking status or BMI (2). Whilst some suggest BGC may be a sequelae of previous infection, in the majority of cases there is no pre-existing history of Bartholin gland disorders (9,10). The squamous cell subtype has, however, been linked to Human Papilloma Virus (HPV) (10-12).
Clinical presentation
The majority of patients are asymptomatic, most often presenting with a painless vulval mass (6). With benign disorders of the Bartholin’s gland being rare in the older female population, peri- or post-menopausal women presenting as such should be suspected as having a potential BGC (13). Less often, a BGC may be associated with non-specific localised symptoms of pain, bleeding, pruritis, or dyspareunia (8). A burning sensation has also been described, provoked by tumour infiltration of perineural spaces (7,14). Whilst bilateral Bartholin’s cancers have being described, this is extremely rare (9).
Diagnosis
Diagnosis of a BGC is delayed in up to 50% of cases (15). This is attributed to the lack of symptoms; as well as frequent mis-diagnosis as a Bartholin’s cyst, abscess—or even endometriosis (2,9,16). When BGC is suspected, however, patients often undergo multi-modal pre-operative imaging. T2-weighted MRI is believed to be the superior modality to identify the primary tumour and accurately determine its dimensions (6,17). MRI will assess for evidence of local invasion into surrounding structures, namely the urethra, bladder and rectum—determining the resectability of the disease (6,8,18). MRI has a sensitivity of 40–50% and specificity of 97–100% for the identification of malignant lymph nodes in vulval cancer (6). CT-TAP and CT-PET can provide additional staging information, assessing for distant lymphadenopathy and metastases (8,17). The definitive diagnosis of a BGC is, however, histopathological (7,9). Pre-operative biopsy is not favoured over formal en-bloc excision—carrying the theoretical risk of capsule breach and hence local seeding; and being insufficient to meet the diagnostic criteria previously described (15):
- Tumour involving the area of the Bartholin’s gland is histologically compatible with origin from the Bartholin’s gland
- Areas of apparent transition from normal elements to neoplastic ones are found in the histological study
- No evidence of primary tumour elsewhere
Histopathology
The Bartholin’s gland proper is composed of columnar epithelium (10). The adjoining ducts are lined by stratified squamous cells, which transform distally into a transitional cell epithelium (10). The majority (50%) of BGC cases are SCCs, arising within these ducts (2). Malignant transformation of glandular tissue into an adenocarcinoma; together with other rarer histological sub-types—adenoid cystic, keratosis gland, transitional cell and undifferentiated carcinomas—together account for the remaining 50% (2-4,12,19). One study reported, of 33 patients with primary BGC, 87.9% with SCC compared to 4 patients (12.1%) developing an adenocarcinoma sub-type (20).
BGC are slow growing and associated with local invasion into the surrounding structures and perineural spaces (2,9). BGC are typically more advanced than non-BGC vulval cancers at presentation—one paper reporting 60.6% versus 35.8% of women as having stage III/IV disease at diagnosis when comparing 33 BGC patients with 396 non-BGC vulval cancer patients (20). This may be attributed in part to the rich vascular and lymphatic networks surrounding the tumour bed facilitating ease of haematogenous and lymphatic dissemination (13). Up to 42% of patients with BGC may have lymph node involvement at diagnosis (2,9,10). One study reported as many as 60.6% presenting with stage III/IV disease (2), with the lungs and bone being the most common sites for distant metastases (9,10).
Management
No consensus exists for the management of BGC, the general approach being based on data extrapolated from that pertaining to other vulval cancers types and cancer of the anus (10,13). For resectable disease—that is, excision with adequate surgical margins deemed feasible without compromise of function of the bladder or rectum—both simple excision and radical vulvectomy have been described (6,8,9). The majority of cases, radical local excision—aiming for margins of >1 cm—is performed. In the context of larger tumours, however, multi-disciplinary surgical input from colorectal and plastics may be required to support partial sphincter resection with defunctioning colostomy for clearance; or flap reconstruction for wound closure. Bilateral inguinofemoral lymphadenectomy is currently recommended by both the British Gynaecological Cancer Society (BGCS) and the Royal College of Obstetricians & Gynaecologist (RCOG) given the propensity of BGCs for early lympho-vascular spread and proximity of the gland to the midline (8,13). No role for sentinel node biopsy (SNB) has been established in the context of a Bartholin’s cancer.
Forty percent of patients with BGC undergoing primary surgery will need adjuvant radiotherapy (20). Radiation reduces rates of local recurrence, especially if positive margins surgical margins are identified (4,7,8,21,22). This is usually administered as interstitial brachy-therapy (6). Chemotherapy agents, including Cisplatin, are commonly used in combination with radiotherapy for the two-fold benefit of firstly enhancing the effects of radiotherapy by inducing radio-sensitisation; whilst also simultaneously inducing direct cytotoxicity (9).
Eight percent of patients with BGC are not surgical candidates at presentation (20). In cases deemed either non-resectable or unfit for surgery, primary radiation or chemoradiation can be administered (6). The intention of neo-adjuvant therapies is to reduce tumour volume, either making future surgery with preservation of function feasible; or to provide symptomatic relief (6,17,23).
Follow-up and prognosis
BGC are typically associated with high rates of disease recurrence (9). A study of 33 patients reported an overall recurrence rate of 40.5% at 5 years (2). The majority (27.3%) of patients experienced vulval relapse; 18.2% in the groin; 9.1% in the pelvic lymph nodes; and 45.4% developed distant metastases of the lung, liver and other sites (2). An important prognostic factor impacting on rates of recurrence is the status of surgical margins (24). One paper reporting recurrence rates of 35% vs. 10% in cases with positive v negative tumour margins respectively (25).
Another prognostic indicator identified as significant is that of lymph node involvement. The overall 5-year survival following BGC is reported as 75%; reducing to 71% in the presence of a single positive inguinal lymph node; and to <20% in the context of multiple positive groin nodes (6,12).
Regular surveillance should be undertaken for patients diagnosed with BGC for a minimum of 5 years (13). Due to post-operative and post-radiotherapy changes, clinical examination may be sub-optimal and thus in the context of this malignancy with tendency for relapse, consideration should be given to interval scanning (7).
Conclusions
This case importantly highlights a rare and aggressive malignancy—and need for a high level of vigilance for BGC in the post-menopausal woman presenting with a gland swelling. We recommend excision of all such tumours in women aged >40 years to avoid a delayed diagnosis due to mis-diagnosis.
BGC typically presents at a more advanced stage, and exhibits higher rates of recurrence and poorer prognosis than that of other non-BGC of the vulva. Primary treatment is most often surgical, incorporating radical excision and bilateral GND. Positive surgical margins are not uncommon due to the deep-seated location of BGCs. For this reason—and common lymphatic involvement at diagnosis—multi-modal treatment with radio/chemotherapy is often indicated. Long-term follow-up incorporating clinical examination and perhaps also serial scanning is recommended.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-612/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-612/coif). HSM serves as an unpaid editorial board member of Translational Cancer Research from September 2021 to August 2023. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Research UK. Available online: https://www.cancerresearchuk.org/. Accessed on 15/11/2021
- Bhalwal AB, Nick AM, Dos Reis R, et al. Carcinoma of the Bartholin Gland: A Review of 33 Cases. Int J Gynecol Cancer 2016;26:785-9. [Crossref] [PubMed]
- Leuchter RS, Hacker NF, Voet RL, et al. Primary carcinoma of the Bartholin gland: a report of 14 cases and review of the literature. Obstet Gynecol 1982;60:361-8. [PubMed]
- Copeland LJ, Sneige N, Gershenson DM, et al. Bartholin gland carcinoma. Obstet Gynecol 1986;67:794-801. [Crossref] [PubMed]
- Cardosi RJ, Speights A, Fiorica JV, et al. Bartholin's gland carcinoma: a 15-year experience. Gynecol Oncol 2001;82:247-51. [Crossref] [PubMed]
- Liebeskind A, Machnicki S, Blackmun D. Case of the month. Applied Radiol 2004;33:49-53. [Crossref]
- Billingsley C, Jackson A, Loreen A. 29-year-old with dyspareunia and vulvar mass: An unusual diagnosis of Bartholin's gland carcinoma. Gynecol Oncol Rep 2018;27:15-8. [Crossref] [PubMed]
- Pellizzon ACA. The adenoid cystic carcinoma of the Bartholin’s gland: a literature review. Appl Cancer Res 2018;38:6. [Crossref]
- Zhan P, Li G, Liu B, et al. Bartholin gland carcinoma: A case report. Oncol Lett 2014;8:849-51. [Crossref] [PubMed]
- Pinn ME, Austin LM, Schomas DA, et al. Case report from Mayo Clinic: Locally advanced Bartholin’s gland carcinoma. Radiol Oncol 2007;41:72-9. [Crossref]
- Felix JC, Cote RJ, Kramer EE, et al. Carcinomas of Bartholin's gland. Histogenesis and the etiological role of human papillomavirus. Am J Pathol 1993;142:925-33. [PubMed]
- Hill DS, Butterfield A. Bartholin’s gland squamous cell carcinoma, a rare vulvar neoplasm. Journal of Diagnostic Medical Sonography 2010;26:296-8. [Crossref]
- Guidelines for the diagnosis and management of vulval carcinoma. British Gynaecological Cancer Society/Royal College of Obstetrician and Gynaecologists 2014.
- Finan MA, Barre G. Bartholin's gland carcinoma, malignant melanoma and other rare tumours of the vulva. Best Pract Res Clin Obstet Gynaecol 2003;17:609-33. [Crossref] [PubMed]
- Chamlian DL, Taylor HB. Primary carcinoma of Bartholin's gland. A report of 24 patients. Obstet Gynecol 1972;39:489-94. [PubMed]
- Akbarzadeh-Jahromi M, Sari Aslani F, Omidifar N, et al. Adenoid Cystic Carcinoma of Bartholin's Gland Clinically Mimics Endometriosis, A Case Report. Iran J Med Sci 2014;39:580-3. [PubMed]
- Massad LS, De Geest K. Multimodality therapy for carcinoma of the Bartholin gland. Gynecol Oncol 1999;75:305-7. [Crossref] [PubMed]
- Downs LS, Ghosh K, Dusenbery KE, et al. Stage IV carcinoma of the Bartholin gland managed with primary chemoradiation. Gynecol Oncol 2002;87:210-2. [Crossref] [PubMed]
- Ouldamer L, Chraibi Z, Arbion F, et al. Bartholin's gland carcinoma: epidemiology and therapeutic management. Surg Oncol 2013;22:117-22. [Crossref] [PubMed]
- Di Donato V, Casorelli A, Bardhi E, et al. Bartholin gland cancer. Crit Rev Oncol Hematol 2017;117:1-11. [Crossref] [PubMed]
- Hwang TL, Hung YC, Chang HW. Adenoid cystic carcinoma of Bartholin's gland. Taiwan J Obstet Gynecol 2012;51:119-20. [Crossref] [PubMed]
- Rosenberg P, Simonsen E, Risberg B. Adenoid cystic carcinoma of Bartholin's gland: a report of five new cases treated with surgery and radiotherapy. Gynecol Oncol 1989;34:145-7. [Crossref] [PubMed]
- López-Varela E, Oliva E, McIntyre JF, et al. Primary treatment of Bartholin's gland carcinoma with radiation and chemoradiation: a report on ten consecutive cases. Int J Gynecol Cancer 2007;17:661-7. [Crossref] [PubMed]
- Şahin Aker S, Cansız Ersöz C, Ortaç F. Adenoid cystic carcinoma of Bartholin's gland diagnosed after lung lobectomy: Review of the literature and a case presentation. Turk J Obstet Gynecol 2020;17:310-3. [Crossref] [PubMed]
- Alsan CI, Vinh-Hung V, Eren F, et al. Adenoid cystic carcinoma of the Bartholin's gland: case report and systematic review of the literature. Eur J Gynaecol Oncol 2011;32:567-72. [PubMed]



