
Identification of risk and prognostic factors for intrahepatic vascular invasion in patients with hepatocellular carcinoma: a population-based study
Introduction
Globally, primary liver cancer is the sixth most common cancer and the third most common cause of cancer-related death (1). Worldwide, approximately 841,000 people are newly diagnosed with liver cancer, and almost 782,000 people die from this disease each year (2). Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver. Patients with HCC are prone to vascular invasion, which is associated with poor prognosis and frequently involves the portal vein, hepatic/vena cava branches, or less frequently, the hepatic arteries (3-5). Two types of hepatic vascular invasion have been defined: macrovascular invasion and microvascular invasion. The former is directly diagnosed by imaging and includes the main portal veins or their branches, hepatic veins, or the inferior vena cava, whereas the latter is defined as the invasion of HCC cells into the microvascular of peritumoral tissues, which is only visible under microscopic examination of specimen after operation (6). Intrahepatic vasculature mainly includes the portal vein or its branches and hepatic veins. Portal vein tumor thrombus (PVTT) is the most common form of intrahepatic vascular invasion (IVI) of HCC and plays a major role in the prognosis and clinical staging of HCC (7,8). PVTT occurs in patients with HCC with a prevalence rate ranging from 44% to 62% (9). In contrast, the incidence of hepatic vein tumor thrombus (HVTT) is relatively low and ranges from 1.4% to 4.9% (10,11). HCC associated with PVTT or HVTT is regarded as advanced HCC, which is related with poor prognosis (12,13). Once PVTT occurs, it progresses rapidly to cause severe complications, such as portal hypertension, hepatocellular jaundice, and intractable ascites. According to previous reports, the median survival time of untreated HCC with PVTT was 2.7–4 months (14). Clinically, PVTT is related to large tumor size, increased tumor number, higher tumor grade, poor Child-Pugh class, and elevated serum alpha-fetoprotein (AFP) (15).
The therapeutic modalities for patients with HCC primarily include liver resection, liver transplantation, radiofrequency ablation, transarterial chemoembolization (TACE), radiotherapy, and systemic therapy. For patients at an early stage [Barcelona Clinic Liver Cancer (BCLC) stage 0 or A], radiofrequency ablation, liver resection, and liver transplantation are the only potential curative treatments (16). TACE is the main treatment method for patients with intermediate-stage tumors (stage B). However, HCC with PVTT is defined as advanced HCC (BCLC stage C) and thus has very limited treatment options. The presence of PVTT is considered a contraindication to surgical resection or TACE regardless of the size of the primary tumor (17,18). Liver transplantation is only suitable for patients with early-stage HCC; however, the shortage of donor livers restricts treatment to some extent (19,20). Although improved survival of HCC patients with major vascular invasion after radiotherapy has been reported, the extreme sensitivity of the liver parenchyma to radiation is still the main obstacle for HCC with IVI (21,22). Sorafenib and lenvatinib are the most representative systemic drugs for the treatment of advanced liver cancer and have been demonstrated to prolong the median survival time by approximately 2–3 months (23).
Considering the poor prognosis and shortage of standard treatment plans, early identification of high-risk patients who are prone to IVI and subsequent active intervention may help achieve greater survival benefits for patients with HCC. It was reported that preoperative neoadjuvant therapies could be used to downstage HCC with PVTT to provide an opportunity for curative surgical resection (24). Postoperative adjuvant TACE has been shown to prolong survival of HCC patients with PVTT (25). A study conducted by Zhang et al. showed that TACE plus sorafenib (TACE-S) was superior to TACE alone in terms of 6-month and 1-year overall survival (OS) and that TACE-S resulted in fewer adverse effects than did TACE alone (26).
In this study, we aimed to identify risk factors for IVI in patients with HCC and further identify prognostic factors for those patients using the Surveillance, Epidemiology, and End Results (SEER) database. Nomograms were also constructed to serve as visual tools to quantify the risk of IVI in patients with HCC and predict the survival of those patients. We present the following article in accordance with the TRIPOD (Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis) reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1912/rc).
Methods
Patient selection
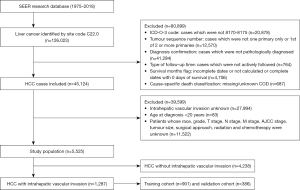
A retrospective case listing from the SEER database from 1975 to 2018 using SEER*Stat software (version 8.3.8; https://seer.cancer.gov/seerstat/) was obtained. Since this study aimed to identify risk factors for IVI in patients with HCC and prognostic factors in HCC patients with IVI, patients in the SEER database from 2010 to 2015 with data on IVI and other definitive information were ultimately included in our study. IVI mainly includes the invasion of the portal vein or its branches and hepatic veins. In this study, the types of vascular invasion included portal vein invasion or its branches, hepatic vein invasion, and unspecified IVI. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The inclusion criteria in the present study were as follows: (I) diagnosed with liver cancer (primary tumor site was the liver; C22.0) between 1975 and 2018; (II) diagnosed with HCC (International Classification of Disease for Oncology, 3rd Edition: 8170, 8171, 8172, 8173, 8174, and 8175) as a primary malignancy between 2010 and 2015; (III) liver cancer was the first primary tumor (tumor sequence number: 1 primary only or the first of 2 or more primaries); (IV) with histopathology confirmed (diagnosis confirmation: positive histology); (V) actively followed up and with definite data on survival time (0 days of survival was excluded); and (VI) with definite information on IVI (CS extension recode: 100, 150, 200, 250, 270, 300, 350, 370, 380, 390, 400, 520, 630, 635, and 638). The exclusion criteria were as follows: (I) missing/unknown COD; (II) age at diagnosis <20 years; (III) unknown race, grade, and tumor size; (IV) unknown T stage, N stage, M stage and American Joint Committee on Cancer (AJCC) stage; and (V) unknown use of radiotherapy, chemotherapy, and surgical approach. In the SEER database, tumor grade was coded as grade 1 (well differentiated), grade 2 (moderately differentiated), grade 3 (poorly differentiated), or grade 4 (undifferentiated), and the tumor, node, metastasis (TNM) staging was conducted based on the 7th edition of the AJCC. As shown in Figure 1, a total of 1,287 HCC patients with IVI were extracted to identify risk factors and prognostic factors. All patients with HCC and IVI were randomly divided into the training (n=901) and validation (n=386) cohorts. For detailed information on selection codes, see Table S1. The raw measurements of 45,124 patients diagnosed with HCC are shown at https://cdn.amegroups.cn/static/public/tcr-22-1912-1.xlsx, and raw measurements of 1,287 patients with HCC and IVI are shown at https://cdn.amegroups.cn/static/public/tcr-22-1912-2.xlsx.
Data elements
The variables used in our study were race, sex, age at diagnosis, grade, T stage, N stage, M stage, AJCC stage, primary tumor size, IVI, AFP, fibrosis score, surgical approach, radiation, chemotherapy, and cause-specific death classification. The surgical approaches for primary HCC included no surgery at the primary site, local tumor destruction, liver resection, and liver transplantation. The primary endpoints of this study were OS and cancer-specific survival (CSS). According to the instructions in SEER database, OS was defined as the time from diagnosis to any cause of death. However, CSS was regarded as the time from diagnosis to death attributed to HCC.
Construction of risk and prognostic nomogram
For risk nomogram construction, the multivariate logistic regression analysis was performed to identify risk factors for IVI in patients with HCC. Those variables that significantly predicted the probability of IVI were used to construct a nomogram for risk evaluation of IVI for patients with HCC.
For prognostic nomogram construction, 1,287 eligible patients were randomly divided into training (n=901) and validation (n=386) cohorts in a ratio of 7:3 (27,28). Univariate and multivariate Cox proportional hazards regression analyses were conducted in the training group, and those variables that significantly related to OS and CSS were used to construct nomograms for predicting 1-, 2-, and 3-year survival rates in HCC patients with IVI.
Validation of risk and prognostic nomogram
The validation of the risk nomogram was performed by using the concordance index (C-index) and calibration curves. The prognostic nomograms were validated in both the training cohort and the validation cohort. The discriminative ability of the nomograms was evaluated by using the C-index and the receiver operating characteristic curve (ROC) and by assessing the area under the curve (AUC) (29,30). The calibration curves were used to assess the predictive accuracy of the nomogram (31). Bootstrapping methods was adopted in 1,000 samples to evaluate the discrimination and calibration of the nomograms. Decision curve analysis (DCA), as a suitable method for evaluating alternative diagnostic and prognostic strategies, was also used to determine whether the nomogram was superior to the AJCC staging system throughout the range of threshold probabilities (32).
Statistical analysis
For this study, HCC patients with IVI were divided into training (n=901) and validation (n=386) cohorts randomly at a ratio of 7:3 by R software (version 4.0.2; The R Foundation for Statistical Computing, Vienna, Austria). Kaplan-Meier method was applied to estimate the OS and CSS. The differences in the significance between the survival curves was assessed by log rank tests. Multivariate logistic regression analysis was adopted to estimate the odds ratio (OR) of the various included risk factors. The multivariable Cox proportional hazards regression model was adopted to examine the hazard ratios (HRs) of the various included prognostic factors. Based on the multivariate logistic regression analysis and multivariate Cox regression analysis, nomograms to predict the risk of IVI of patients with HCC and to estimate the OS and CSS of HCC patients with IVI were developed, respectively, by incorporating the various independent risk and prognostic factors. The predictive ability of risk nomogram was assessed by using the C-index and calibration curves. The discriminative ability of the prognostic nomograms was evaluated by using the C-index and the ROC curve. Additionally, the 1-, 2-, and 3-year calibration curve analysis and DCA was applied to assess the nomograms. All tests were 2-sided, and a value of P value of less than 0.05 was considered statistically significant. All statistical analyses were all performed with SPSS 26.0 (IBM Corp., Armonk, NY, USA) and R software (version 4.0.2).
Results
Baseline characteristics of the population study
A total of 5,525 patients with HCC with definitive information on IVI were included in our study, and of these, 1,287 (23.3%) had IVI at initial diagnosis and 4,238 cases (76.7%) did not. Table 1 shows the demographic and clinicopathologic characteristics of the study population. According to the data characteristics of the table, we found that the proportions of HCC patients with IVI stratified by age, race, pathological grade, T stage, N stage, M stage, primary tumor size, AFP, and fibrosis score were significantly different. In addition, patients with HCC who were younger, non-White, higher pathological grade (grade III and IV), higher N stage (N1), higher M stage (M1), larger primary tumor size (>5 cm), elevated AFP, and F0 (no fibrosis or mild to moderate fibrosis) had a higher incidence of IVI. We then evaluated a total of 1,287 HCC patients with IVI. Patients were divided into a training cohort and validation cohort in a ratio of 7:3 by the split-sample method. Table 2 presents the detailed information for the training and validation cohorts, which were found to be comparable.
Table 1
| Variables | Without IVI, n (%) | With IVI, n (%) | P value |
|---|---|---|---|
| Age (year) | 0.014 | ||
| <65 | 2,361 (55.7) | 767 (59.6) | |
| ≥65 | 1,877 (44.3) | 520 (40.4) | |
| Race | 0.010 | ||
| White | 2,860 (67.5) | 814 (63.2) | |
| Black | 522 (12.3) | 193 (15.0) | |
| Other | 856 (20.2) | 280 (21.8) | |
| Sex | 0.060 | ||
| Male | 3,232 (76.3) | 1,014 (78.8) | |
| Female | 1,006 (23.7) | 273 (21.2) | |
| Grade | <0.001 | ||
| I | 1,420 (33.5) | 213 (16.6) | |
| II | 2,100 (49.6) | 657 (51.0) | |
| III | 658 (15.5) | 395 (30.7) | |
| IV | 60 (1.4) | 22 (1.7) | |
| T stage | <0.001 | ||
| T1 | 3,123 (73.7) | 0 | |
| T2 | 691 (16.3) | 584 (45.4) | |
| T3a | 424 (10.0) | 81 (6.3) | |
| T3b | 0 | 622 (48.3) | |
| N stage | <0.001 | ||
| N0 | 4,106 (96.9) | 1,132 (88.0) | |
| N1 | 132 (3.1) | 155 (12.0) | |
| M stage | <0.001 | ||
| M0 | 4,022 (94.9) | 1,110 (86.2) | |
| M1 | 216 (5.1) | 177 (13.8) | |
| Tumor size (cm) | <0.001 | ||
| ≤5 | 2,755 (65.0) | 451 (35.0) | |
| >5 | 1,483 (35.0) | 836 (65.0) | |
| AFP | <0.001 | ||
| Normal | 1,312 (31.0) | 251 (19.5) | |
| Elevated | 2,120 (50.0) | 843 (65.5) | |
| Unknown | 806 (19.0) | 193 (15.0) | |
| Fibrosis score | <0.001 | ||
| F0 | 438 (10.3) | 152 (11.8) | |
| F1 | 1,094 (25.8) | 245 (19.0) | |
| Unknown | 2,706 (63.9) | 890 (69.2) | |
| Radiation | <0.001 | ||
| No | 3,966 (93.6) | 1,141 (88.7) | |
| Yes | 272 (6.4) | 146 (11.3) | |
| Chemotherapy | <0.001 | ||
| No | 2,870 (67.7) | 790 (61.4) | |
| Yes | 1,368 (32.3) | 497 (38.6) | |
| Surgical approach | <0.001 | ||
| No surgery | 1,683 (39.7) | 675 (52.4) | |
| Local tumor destruction | 637 (15.0) | 43 (3.3) | |
| Liver resection | 1,308 (30.9) | 486 (37.8) | |
| Liver transplantation | 610 (14.4) | 83 (6.4) |
HCC, hepatocellular carcinoma; IVI, intrahepatic vascular invasion; AFP, alpha-fetoprotein; Other, includes American Indian/Alaskan native and Asian/Pacific Islander; F0, equivalent to Ishak score 0–4 (no fibrosis or mild to moderate fibrosis); F1, equivalent to Ishak score 5–6 (severe fibrosis or cirrhosis).
Table 2
| Characteristics | Total cohort (n=1,287), n (%) | Training cohort (n=901, 70.0%), n (%) | Validation cohort (n=386, 30.0%), n (%) | P value |
|---|---|---|---|---|
| Age (year) | 0.624 | |||
| <65 | 767 (59.6%) | 533 (59.2%) | 234 (60.6%) | |
| ≥65 | 520 (40.4%) | 368 (40.8%) | 152 (39.4%) | |
| Race | 0.870 | |||
| White | 814 (63.2%) | 569 (63.2%) | 245 (63.5%) | |
| Black | 193 (15.0%) | 138 (15.3%) | 55 (14.2%) | |
| Other | 280 (21.8%) | 194 (21.5%) | 86 (22.3%) | |
| Sex | 0.642 | |||
| Male | 1,014 (78.8%) | 713 (79.1%) | 301 (78.0%) | |
| Female | 273 (21.2%) | 188 (20.9%) | 85 (22.0%) | |
| Grade | 0.972 | |||
| I | 213 (16.6%) | 151 (16.8%) | 62 (16.1%) | |
| II | 657 (51.0%) | 460 (51.1%) | 197 (51.0%) | |
| III | 395 (30.7%) | 274 (30.4%) | 121 (31.3%) | |
| IV | 22 (1.7%) | 16 (1.8%) | 6 (1.6%) | |
| T stage | 0.847 | |||
| T2 | 584 (45.4%) | 412 (45.7%) | 172 (44.6%) | |
| T3a | 81 (6.3%) | 58 (6.4%) | 23 (6.0%) | |
| T3b | 622 (48.3%) | 431 (47.8%) | 191 (49.5%) | |
| N stage | 0.639 | |||
| N0 | 1,132 (88.0%) | 795 (88.2%) | 337 (87.3%) | |
| N1 | 155 (12.0%) | 106 (11.8%) | 49 (12.7%) | |
| M stage | 0.988 | |||
| M0 | 1,110 (86.2%) | 777 (86.2%) | 333 (86.3%) | |
| M1 | 177 (13.8%) | 124 (13.8%) | 53 (13.7%) | |
| Tumor size (cm) | 0.060 | |||
| ≤5 | 451 (35.0%) | 301 (33.4%) | 150 (38.9%) | |
| >5 | 836 (65.0%) | 600 (66.6%) | 236 (61.1%) | |
| AFP | 0.090 | |||
| Normal | 251 (19.5%) | 190 (21.1%) | 61 (15.8%) | |
| Elevated | 843 (65.5%) | 578 (64.2%) | 265 (68.7%) | |
| Unknown | 193 (15.0%) | 133 (14.8%) | 60 (15.5%) | |
| Fibrosis score | 0.248 | |||
| F0 | 152 (11.8%) | 98 (10.9%) | 54 (14.0%) | |
| F1 | 245 (19.0%) | 170 (18.9%) | 75 (19.4%) | |
| Unknown | 890 (69.2%) | 633 (70.3%) | 257 (66.6%) | |
| Radiation | 0.880 | |||
| No | 1,141 (88.7%) | 798 (88.6%) | 343 (88.9%) | |
| Yes | 146 (11.3%) | 103 (11.4%) | 43 (11.1%) | |
| Chemotherapy | 0.449 | |||
| No | 790 (61.4%) | 547 (60.7%) | 243 (63.0%) | |
| Yes | 497 (38.6%) | 354 (39.3%) | 143 (37.0%) | |
| Surgical approach | 0.234 | |||
| No surgery | 675 (52.4%) | 487 (54.1%) | 188 (48.7%) | |
| Local tumor destruction | 43 (3.3%) | 30 (3.3%) | 13 (3.4%) | |
| Liver resection | 486 (37.8%) | 324 (36.0%) | 162 (42.0%) | |
| Liver transplantation | 83 (6.4%) | 60 (6.7%) | 23 (6.0%) | |
| Median follow-up time [months, 25th-75th percentile] | 12 [3–50] | 11 [3–50] | 14 [4–51] | 0.828 |
AFP, alpha-fetoprotein; Other, includes American Indian/Alaskan native and Asian/Pacific Islander; F0, equivalent to Ishak score 0–4 (no fibrosis or mild to moderate fibrosis); F1, equivalent to Ishak score 5–6 (severe fibrosis or cirrhosis).
Survival analysis of patients in total cohort
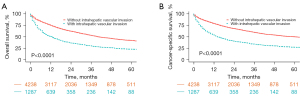
The median OS of all patients with HCC was 20 months [interquartile range (IQR) 7–39 months]. The median OS of HCC patients with and without IVI were 11 months (IQR 3–28 months) and 22 months (IQR 11–42 months), respectively. The median OS of patients with and without surgery was 30 months (IQR 17–50 months) and 8 months (IQR 2–20 months), respectively. The 1-, 2-, and 3-year OS rates of all patients were 68.6%, 55.7%, and 47.0%, respectively, whereas they were 49.9%, 35.9%, and 29.7%, respectively, in patients with IVI and 74.2%, 61.6%, and 52.3%, respectively, in those without IVI. The results revealed that HCC patients with IVI had a dismal survival rate. Figure 2 shows that HCC patients with IVI have significantly worse OS and CSS compared to those without IVI.
Identification of risk factors in HCC patients with IVI
Results of the multivariate logistic regression analysis (as shown in Table 3) indicated that grade II (OR: 1.89; 95% CI: 1.58–2.25; P<0.001), grade III (OR: 2.76; 95% CI: 2.25–3.38; P<0.001), N1 stage (OR: 2.21; 95% CI: 1.69–2.90; P<0.001), M1 stage (OR: 1.47; 95% CI: 1.16–1.87; P=0.002), larger (>5 cm) tumor size (OR: 3.03; 95% CI: 2.64–3.49; P<0.001), and elevated AFP (OR: 1.61; 95% CI: 1.36–1.90; P<0.001) were potentially significant high-risk factors compared with grade I, N0 stage, M0 stage, small (≤5 cm) primary tumor size, and normal AFP. However, age ≥65 years (OR: 0.76; 95% CI: 0.66–0.87; P<0.001) was significantly associated with a lower risk of IVI.
Table 3
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Age (year) | <0.001 | ||
| <65 | Reference | ||
| ≥65 | 0.76 | 0.66–0.87 | <0.001 |
| Race | 0.278 | ||
| White | Reference | ||
| Black | 1.15 | 0.94–1.40 | 0.166 |
| Other | 1.10 | 0.93–1.30 | 0.284 |
| Grade | <0.001 | ||
| I | Reference | ||
| II | 1.89 | 1.58–2.25 | <0.001 |
| III | 2.76 | 2.25–3.38 | <0.001 |
| IV | 1.42 | 0.84–2.42 | 0.194 |
| N stage | <0.001 | ||
| N0 | Reference | ||
| N1 | 2.21 | 1.69–2.90 | <0.001 |
| M stage | 0.001 | ||
| M0 | Reference | ||
| M1 | 1.47 | 1.16–1.87 | 0.002 |
| Tumor size (cm) | <0.001 | ||
| ≤5 | Reference | ||
| >5 | 3.03 | 2.64–3.49 | <0.001 |
| AFP | <0.001 | ||
| Normal | Reference | ||
| Elevated | 1.61 | 1.36–1.90 | <0.001 |
| Unknown | 1.10 | 0.88–1.37 | 0.406 |
| Fibrosis score | 0.198 | ||
| F0 | Reference | ||
| F1 | 1.07 | 0.87–1.33 | 0.527 |
| Unknown | 0.87 | 0.74–1.04 | 0.125 |
IVI, intrahepatic vascular invasion; HCC, hepatocellular carcinoma; OR, odds radio; CI, confidence interval; Other, includes American Indian/Alaskan native and Asian/Pacific Islander; AFP, alpha-fetoprotein; F0, equivalent to Ishak score 0–4 (no fibrosis or mild to moderate fibrosis); F1, equivalent to Ishak score 5–6 (severe fibrosis or cirrhosis).
Identification of prognostic factors in the training cohort
As shown in Table 4, we identified 8 identical independent prognostic factors for OS and CSS in the training cohort based on the univariate and multivariate Cox proportional hazards regression analysis. For CSS prediction, female gender (HR: 0.78; 95% CI: 0.63–0.96; P=0.020), grade III (HR: 1.75; 95% CI: 1.36–2.25; P<0.001), T3b stage (HR: 1.82; 95% CI: 1.45–2.30; P<0.001), N1 stage (HR: 1.31; 95% CI: 1.02–1.68; P=0.033), M1 stage (HR: 1.54; 95% CI: 1.23–1.94; P<0.001), larger (>5 cm) primary tumor size (HR: 1.25; 95% CI: 1.02–1.54; P=0.033), elevated AFP (HR: 1.41; 95% CI: 1.13–1.77; P=0.003), local tumor destruction (HR: 0.56; 95% CI: 0.35–0.91; P=0.019), liver resection (HR: 0.36; 95% CI: 0.28–0.46; P<0.001), and liver transplantation (HR: 0.13; 95% CI: 0.07–0.23; P<0.001) were all significantly associated with CSS in HCC patients with IVI. For grade II, grade IV, and T3a stage, although no significant difference was observed in prognosis, a tendency toward poor CSS was seen compared with grade I and T2 stage. The same conclusion was found for OS prediction, which is also shown in Table 4.
Table 4
| Variables | OS | CSS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age (year) | |||||||||||
| <65 | Reference | Reference | Reference | Reference | |||||||
| ≥65 | 1.18 (1.01–1.38) | 0.041 | 1.16 (0.99–1.37) | 0.070 | 1.15 (0.98–1.36) | 0.098 | |||||
| Race | |||||||||||
| White | Reference | Reference | Reference | Reference | |||||||
| Black | 0.93 (0.75–1.16) | 0.528 | 0.94 (0.75–1.18) | 0.572 | 0.94 (0.75–1.19) | 0.626 | 0.93 (0.74–1.18) | 0.566 | |||
| Other | 0.74 (0.60–0.90) | 0.003 | 0.88 (0.72–1.09) | 0.239 | 0.75 (0.61–0.93) | 0.008 | 0.90 (0.73–1.12) | 0.354 | |||
| Sex | |||||||||||
| Male | Reference | Reference | Reference | Reference | |||||||
| Female | 0.73 (0.60–0.90) | 0.002 | 0.75 (0.61–0.92) | 0.005 | 0.75 (0.61–0.92) | 0.006 | 0.78 (0.63–0.96) | 0.020 | |||
| Grade | |||||||||||
| I | Reference | Reference | Reference | Reference | |||||||
| II | 0.78 (0.63–0.96) | 0.022 | 1.10 (0.88–1.38) | 0.409 | 0.75 (0.60–0.94) | 0.013 | 1.08 (0.85–1.36) | 0.536 | |||
| III | 1.26 (1.00–1.58) | 0.047 | 1.79 (1.41–2.28) | <0.001 | 1.25 (0.99–1.59) | 0.062 | 1.75 (1.36–2.25) | <0.001 | |||
| IV | 1.07 (0.60–1.91) | 0.809 | 1.22 (0.68–2.21) | 0.501 | 0.89 (0.47–1.71) | 0.730 | 1.03 (0.53–2.00) | 0.934 | |||
| T stage | |||||||||||
| T2 | Reference | Reference | Reference | Reference | |||||||
| T3a | 1.72 (1.23–2.41) | 0.002 | 1.30 (0.91–1.85) | 0.154 | 1.92 (1.36–2.73) | <0.001 | 1.42 (0.98–2.06) | 0.062 | |||
| T3b | 3.53 (2.97–4.19) | <0.001 | 1.65 (1.33–2.05) | <0.001 | 3.88 (3.23–4.67) | <0.001 | 1.82 (1.45–2.30) | <0.001 | |||
| N stage | |||||||||||
| N0 | Reference | Reference | Reference | Reference | |||||||
| N1 | 2.92 (2.33–3.65) | <0.001 | 1.36 (1.07–1.73) | 0.012 | 2.89 (2.29–3.66) | <0.001 | 1.31 (1.02–1.68) | 0.033 | |||
| M stage | |||||||||||
| M0 | Reference | Reference | Reference | Reference | |||||||
| M1 | 2.94 (2.39–3.62) | <0.001 | 1.55 (1.24–1.93) | <0.001 | 2.99 (2.41–3.70) | <0.001 | 1.54 (1.23–1.94) | <0.001 | |||
| Tumor size (cm) | |||||||||||
| ≤5 | Reference | Reference | Reference | Reference | |||||||
| >5 | 1.86 (1.56–2.21) | <0.001 | 1.24 (1.02–1.51) | 0.029 | 1.94 (1.61–2.34) | <0.001 | 1.25 (1.02–1.54) | 0.033 | |||
| AFP | |||||||||||
| Normal | Reference | Reference | Reference | Reference | |||||||
| Elevated | 1.54 (1.26–1.90) | <0.001 | 1.39 (1.12–1.73) | 0.003 | 1.56 (1.25–1.94) | <0.001 | 1.41 (1.13–1.77) | 0.003 | |||
| Unknown | 1.58 (1.21–2.06) | 0.001 | 1.56 (1.19–2.06) | 0.001 | 1.61 (1.21–2.13) | 0.001 | 1.59 (1.19–2.12) | 0.002 | |||
| Fibrosis score | |||||||||||
| F0 | Reference | Reference | Reference | Reference | |||||||
| F1 | 1.36 (1.00–1.87) | 0.052 | 1.41 (1.02–1.94) | 0.059 | 1.33 (0.95–1.85) | 0.096 | 1.32 (0.94–1.85) | 0.115 | |||
| Unknown | 1.65 (1.26–2.16) | <0.001 | 1.29 (0.97–1.70) | 0.078 | 1.68 (1.26–2.23) | <0.001 | 1.28 (0.95–1.71) | 0.103 | |||
| Surgical approach | |||||||||||
| No surgery | Reference | Reference | Reference | Reference | |||||||
| Local tumor destruction | 0.34 (0.22–0.53) | <0.001 | 0.51 (0.32–0.81) | <0.001 | 0.35 (0.22–0.56) | <0.001 | 0.56 (0.35–0.91) | 0.019 | |||
| Liver resection | 0.25 (0.21–0.30) | <0.001 | 0.35 (0.27–0.44) | 0.004 | 0.25 (0.21–0.31) | <0.001 | 0.36 (0.28–0.46) | <0.001 | |||
| Liver transplantation | 0.14 (0.09–0.21) | <0.001 | 0.19 (0.12–0.31) | <0.001 | 0.09 (0.05–0.15) | <0.001 | 0.13 (0.07–0.23) | <0.001 | |||
| Radiation | |||||||||||
| No | Reference | Reference | Reference | Reference | |||||||
| Yes | 1.05 (0.82–1.34) | 0.716 | 1.12 (0.87–1.44) | 0.368 | |||||||
| Chemotherapy | |||||||||||
| No | Reference | Reference | Reference | Reference | |||||||
| Yes | 1.10 (0.94–1.29) | 0.228 | 1.14 (0.97–1.34) | 0.126 | |||||||
OS, overall survival; CSS, cancer-specific survival; IVI, intrahepatic vascular invasion; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; Other, includes American Indian/Alaskan native and Asian/Pacific Islander; AFP, alpha-fetoprotein; F0, equivalent to Ishak score 0–4 (no fibrosis or mild to moderate fibrosis); F1, equivalent to Ishak score 5–6 (severe fibrosis or cirrhosis).
Construction and validation of the nomogram risk model
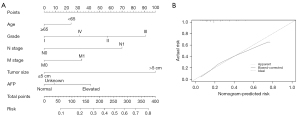
A nomogram for risk assessment of IVI was constructed based on the above significant risk factors. We excluded 3 variables related to therapy, which were not suitable as risk predictors. The nomogram showed that tumor size contributed most to the risk evaluation, followed by grade, N stage, AFP, M stage, and age (Figure 3A). Harrell’s C-index statistic of the nomogram for risk prediction was 0.730 (95% CI: 0.715–0.745), which indicated good predictive efficiency in evaluating the risk of IVI for patients with HCC. The calibration plots showed a consistency between the nomogram-predicted risk and the actual risk (Figure 3B).
Construction and validation of the nomogram prognostic model
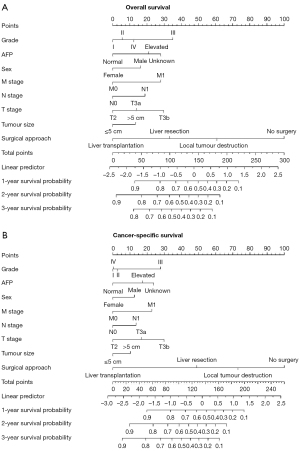
Two prognostic nomograms based on the selected prognostic factors from the training cohort were also developed for the prediction of OS and CSS at 1-, 2-, and 3-year according to the multivariate Cox proportional hazards regression analysis. Both nomograms demonstrated that the surgical approach contributed the most to prognosis, followed by T stage, grade, AFP, M stage, N stage, sex, and tumor size (Figure 4). Each level of every variable was assigned a score on the points scale. The total score was obtained by adding the scores for each selected variable. The prediction corresponding to the total score were regarded as the 1-, 2-, and 3-year OS and CSS of each patient.
The 2 prognostic nomograms were validated internally in the training cohort and externally in the validation cohort. The C-indices based on the nomogram were 0.762 (95% CI: 0.745–0.779) for OS prediction and 0.770 (95% CI: 0.753–0.787) for CSS prediction in the training cohort. In the validation cohort, the C-indices were 0.779 (95% CI: 0.752–0.806) and 0.795 (95% CI: 0.768–0.822) for OS prediction and CSS prediction, respectively, which further indicated a good predictive accuracy for the OS and CSS of patients with HCC and IVI. Moreover, our prognostic model was found to be superior to the traditional AJCC staging system because it demonstrated better discriminative ability in both the training (1-year AUC: 0.854 vs. 0.752; 2-year AUC: 0.859 vs. 0.743; 3-year AUC: 0.857 vs. 0.731; Figure 5A) and validation (1-year AUC: 0.853 vs. 0.746; 2-year AUC: 0.854 vs. 0.750; 3-year AUC: 0.851 vs. 0.729; Figure 5B) cohorts for 1-, 2-, and 3-year OS. The same trend was observed for the 1-, 2-, and 3-year CSS in both the training cohort (Figure 5C) and the validation cohort (Figure 5D).

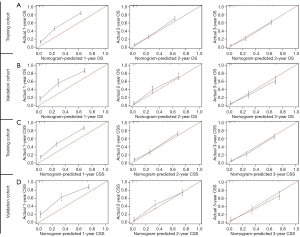
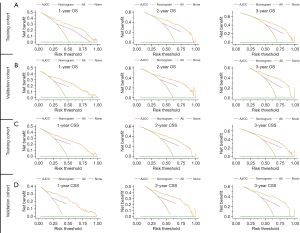
The calibration plots exhibited a good agreement between predicted and observed survival for the 1-, 2-, and 3-year OS in the training (Figure 6A) and validation cohorts (Figure 6B). Likewise, the calibration plots for CSS at different time points also showed excellent consistency with the survival predicted by the nomogram and observed survival in both the training cohort (Figure 6C) and validation cohort (Figure 6D). These findings suggested that the 2 prognostic nomograms were reliable to some extent. As shown in Figure 7, DCA showed that 2 prognostic nomograms had good clinical validity in predicting the 1-, 2-, and 3-year OS and CSS of HCC patients with IVI due to the wide field of threshold probability. Furthermore, a preferable net benefit was also obtained with the formulated nomogram compared with the AJCC staging system at different time points, which indicated the favorable clinical utility of the 2 nomograms
Discussion
HCC is a highly invasive primary malignant tumor that is prone to intrahepatic metastasis via vascular invasion (33). Patients with IVI have a poor prognosis, including unilateral or main PVTT and HVTT. PVTT is associated with a poor prognosis because it is closely related to intrahepatic metastasis and tumor recurrence. Under optimal supportive treatment, the medial survival time of patients with PVTT is only 2–4 months (34,35). A retrospective study showed that the disease control rate of patients with HCC and PVTT was about 33.3% (36). HVTT usually coexists with PVTT in patients with HCC, which increases the difficulty in evaluating the prognosis of HVTT alone (11,37). In our study, the median OS of patients with IVI was 11 months, and the 1-, 2-, and 3-year OS rates were 49.9%, 35.9%, and 29.7% for patients with IVI, respectively, which revealed a poor prognosis of HCC patients with IVI. Therefore, it is important to explore the predictors of IVI from HCC for clinical decision-making. A previous study showed that cirrhosis, a serum alkaline phosphatase (ALP) level >100 IU/L, tumor size >8 cm, incomplete tumor capsule, and adjacent organ invasion were independent risk factors for PVTT in HCC according to a multivariate analysis (38). In addition, it was reported that larger tumor size (>5 cm) and higher serum AFP level were strong preoperative predictors of vascular invasion including portal venous invasion (39). Patients with PVTT had bigger tumors and higher AFP levels at the time of diagnosis compared to those without PVTT (40). It was also reported that the grade of tumor differentiation is closely related to vascular invasion and proliferation (41,42). The expression of angiogenic factors in poorly differentiated HCC was increased, and the microvessel density was high, which may at least partially explain why poor differentiation is more closely related to vascular invasion (41,43).
In our study, we concluded that younger age (<65 years), grade II and III tumors, N1 stage, M1 stage, larger primary tumor size (>5 cm), and elevated AFP were significant predictors of high potential of IVI from HCC. Our research further concluded that HCC patients with IVI with grade III, T3b stage, N1 stage, M1 stage, larger (>5 cm) primary tumor size, elevated AFP, and no surgery were significantly associated with shorter survival (OS and CSS) than those with grade I, T2 stage, N0 stage, M0 stage, small (≤5 cm) tumor size, normal AFP, and surgery. Female patients with HCC and IVI seemed to have a better survival compared to their male counterparts. Higher histologic grade refers to poorly differentiated or undifferentiated tumor, which indicates higher malignancy of the tumor. According to the AJCC staging system, the T stage is mainly classified according to tumor size, vascular invasion, and the number of primary tumor lesions. In our study, we found that the T stage was significantly associated with the survival of HCC patients with IVI. The higher the T stage was, the higher the likelihood of tumor invasion and metastasis, which may partially explain why a higher T stage contributed to worse survival. Consistent with our results, findings of other studies show lymph node metastasis in patients with HCC to be closely related to a lower survival rate, with vascular invasion or extrahepatic metastasis indicating advanced-stage cancer and poor prognosis (44,45).
Serum AFP is currently the most widely used biomarker for HCC screening, early diagnosis, and efficacy and prognostic evaluation (46). The expression level of AFP is closely related to the occurrence and development of HCC. High serum levels of AFP usually indicate a high risk of HCC development and a poor prognosis (47). AFP can promote the metastasis of HCC cells by promoting the expression of metastasis-related genes, which can play a key role in the invasion and distant metastasis of cancer cells (48). Our research found that patients diagnosed with HCC who had an elevated AFP level were more likely to have IVI than were patients with a normal AFP level, which is consistent with a previous report (49). We also concluded that HCC patients with IVI with elevated AFP were associated with shorter survival (OS and CSS).
Notably, Zhang et al. found that young patients with HCC were more often associated with aggressive tumor behavior with larger tumor size and higher serum AFP level and that these patients had more unfavorable pathological characteristics including bigger lesion size and portal vein invasion (50). The presence of macrovascular invasion in HCC has been associated with younger age at diagnosis, elevated AFP levels, and large tumor size (51). In agreement with previous studies, our research found that patients with HCC with elevated AFP level were more likely to develop IVI compared to patients with a normal AFP level. Moreover, IVI is more common in patients with HCC younger than 65 years old. The phenomenon that young patients with HCC are more prone to IVI may involve complex mechanisms, which were not elucidated in our study. Younger age and elevated AFP are potential risk factors for IVI. Due our study having a different focus, we did not explore the correlation between age and AFP. Based on the above statements, our study partially explained the association of patients older than 65 years with a lower risk of IVI.
In patients with HCC with macrovascular invasion, including PVTT or HVTT, the optimal treatment strategy has not yet been established. Although sorafenib is approved as a first-line therapy for patients with advanced HCC, the therapeutic benefits of sorafenib in prolonging survival are limited (52). Currently, no adjuvant chemotherapies are approved as a standard treatment for postoperative patients because of their controversial effects, which highlights the need for better treatment strategies (53,54). In the current study, it was evident that patients with IVI who received chemotherapy had better survival. Nevertheless, due to the lack of detailed chemotherapy strategy information in our data set, we cannot compare the effects of different chemotherapy regimens on survival. Curative-intent surgery is usually technically challenging, and with a high tumor recurrence rate, surgical resection and liver transplantation are also contraindicated in HCC with IVI (55,56). However, our research showed that the prognosis of patients receiving surgical treatment was more favorable than that of patients receiving nonsurgical treatment in the overall sample. Two large-scale studies of patients diagnosed with HCC with PVTT from China and Japan showed that the median survival times of the surgery group were much longer than those of their counterparts who did not undergo surgery (57,58). However, the therapeutic effects of surgical treatment have only been confirmed in tumor thrombi not involving the main portal vein or the superior mesenteric vein (59).
To identify patients with HCC at high risk for IVI and to predict their survival, we established nomograms and then validated their predictive accuracy. The results showed that the models we constructed had good prediction ability and could be used as personalized prediction tools for clinical decision-making. The diagnosis of HCC is a prerequisite for the diagnosis of portal vein thrombosis. We suggested early monitoring of high-risk populations for HCC, including regular AFP detection. The nomogram we developed for risk evaluation of IVI from patients with HCC showed that younger age (<65 years), grade II and III tumors, N1 stage, M1 stage, larger primary tumor size (>5 cm), and elevated AFP were high risk factors for the presence of IVI in patients with HCC. Therefore, we suggest that regular detection of tumor markers and abdominal imaging examinations should be considered to diagnose IVI early for patients with HCC characterized by younger age (<65 years), grade II and III tumors, N1 stage, M1 stage, larger primary tumor size (>5 cm), and elevated AFP to aid in the early detection of IVI and the early determination of treatment options. Furthermore, the nomograms we constructed for OS prediction and CSS prediction for HCC patients with IVI could also help clinicians to predict the precise likelihood of survival at different timepoints and to screen patients at high risk of early death. Based on the predicted survival, we could make more appropriate treatment regimens for individual HCC patient with IVI.
To our knowledge, this is the first population-based study to focus on establishing clinical prediction models to evaluate the risk of IVI for patients with HCC and to predict survival for patients with HCC diagnosed with IVI. We evaluated the clinical applicability of the nomograms for predicting OS and CSS by comparing them to the AJCC stage. To our delight, our nomograms had better discriminability and accuracy for predicting 1-, 2-, and 3-year OS and CSS, respectively. In addition, our nomograms predicted survival with higher accuracy and more net benefit. We believe our models may be useful tools for helping clinicians quantify the risk of IVI, estimate survival, and thus make appropriate treatment strategies for individual patients.
However, the present work still had some limitations. First, this was a retrospective study in which selection bias was inevitable. Second, information on detailed therapeutic modalities such as TACE for patients with HCC with vascular invasion was not available in the SEER database. Third, we could not analyze the risk predictors and prognostic factors of PVTT or HVTT alone because the SEER database does not classify IVI at length. Fourth, the SEER database lacked specific information on HCC etiology, functional liver status, performance status, total tumor volume, and Child-Pugh scores, which may be confounding factors in this study and might have influenced the results. In summary, more detailed data are needed in the future.
Conclusions
Our study showed that patients with HCC characterized by younger age (<65 years), grade II and III tumors, N1 stage, M1 stage, larger primary tumor size (>5 cm), and elevated AFP were more likely to have IVI. In addition, male sex, grade III, T3b stage, N1 stage, M1 stage, larger (>5 cm) primary tumor size, elevated AFP, and nonsurgical treatment were significantly associated with shorter survival (OS and CSS) compared with female sex, grade I, T2 stage, N0 stage, M0 stage, small (≤5 cm) tumor size, normal AFP, and surgical treatment. Grade II, grade IV, and T3a stage showed a tendency toward poor prognosis of OS and CSS, and should be given greater attention. The nomograms we constructed may be individualized and convenient tools to identify IVI at initial diagnosis and may be used to make a prognostic assessment for IVI in patients with HCC.
Acknowledgments
We are grateful to all the patients and individuals in the study who made this work possible.
Funding: This work was supported by the Spark Research Fund (No. HYDSYXH201904 to BZ) and the General Research Project (No. HYDSYJQ201605 to FH) from The Fourth Affiliated Hospital of Harbin Medical University and by the Natural Scientific Foundation of Heilongjiang Province (No. H2018028 to FH and No. LH2019H018 to BZ).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1912/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1912/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1912/coif). BZ was funded by the Spark Research Fund (No. HYDSYXH201904) from The Fourth Affiliated Hospital of Harbin Medical University and by the Natural Scientific Foundation of Heilongjiang Province (No. LH2019H018). FH was funded by the General Research Project (No. HYDSYJQ201605) from The Fourth Affiliated Hospital of Harbin Medical University and by the Natural Scientific Foundation of Heilongjiang Province (No. H2018028). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Pesi B, Ferrero A, Grazi GL, et al. Liver resection with thrombectomy as a treatment of hepatocellular carcinoma with major vascular invasion: results from a retrospective multicentric study. Am J Surg 2015;210:35-44. [Crossref] [PubMed]
- Marshall A, Alexander G. Vascular invasion leaves its mark in hepatocellular carcinoma. J Hepatol 2011;55:1174-5. [Crossref] [PubMed]
- Chen CK, Yang CY, Hua KT, et al. Leukocyte cell-derived chemotaxin 2 antagonizes MET receptor activation to suppress hepatocellular carcinoma vascular invasion by protein tyrosine phosphatase 1B recruitment. Hepatology 2014;59:974-85. [Crossref] [PubMed]
- Lee S, Kang TW, Song KD, et al. Effect of Microvascular Invasion Risk on Early Recurrence of Hepatocellular Carcinoma After Surgery and Radiofrequency Ablation. Ann Surg 2021;273:564-71. [Crossref] [PubMed]
- Li SH, Wei W, Guo RP, et al. Long-term outcomes after curative resection for patients with macroscopically solitary hepatocellular carcinoma without macrovascular invasion and an analysis of prognostic factors. Med Oncol 2013;30:696. [Crossref] [PubMed]
- Li SH, Guo ZX, Xiao CZ, et al. Risk factors for early and late intrahepatic recurrence in patients with single hepatocellular carcinoma without macrovascular invasion after curative resection. Asian Pac J Cancer Prev 2013;14:4759-63. [Crossref] [PubMed]
- Liu PH, Huo TI, Miksad RA. Hepatocellular Carcinoma with Portal Vein Tumor Involvement: Best Management Strategies. Semin Liver Dis 2018;38:242-51. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Yamamoto S, et al. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol 2014;61:583-8. [Crossref] [PubMed]
- Zhang XP, Liu YC, Chen ZH, et al. Postoperative Adjuvant Transarterial Chemoembolization Improves Outcomes of Hepatocellular Carcinoma Associated with Hepatic Vein Invasion: A Propensity Score Matching Analysis. Ann Surg Oncol 2019;26:1465-73. [Crossref] [PubMed]
- Luo F, Li M, Ding J, et al. The Progress in the Treatment of Hepatocellular Carcinoma With Portal Vein Tumor Thrombus. Front Oncol 2021;11:635731. [Crossref] [PubMed]
- Mähringer-Kunz A, Meyer FI, Hahn F, et al. Hepatic vein tumor thrombosis in patients with hepatocellular carcinoma: Prevalence and clinical significance. United European Gastroenterol J 2021;9:590-7. [Crossref] [PubMed]
- Lu J, Zhang XP, Zhong BY, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol 2019;4:721-30. [Crossref] [PubMed]
- Connolly GC, Chen R, Hyrien O, et al. Incidence, risk factors and consequences of portal vein and systemic thromboses in hepatocellular carcinoma. Thromb Res 2008;122:299-306. [Crossref] [PubMed]
- Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int 2019;13:125-37. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. Erratum in: J Hepatol 2019;70:817. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94.e3; quiz e14-5. [Crossref] [PubMed]
- Akoad ME, Pomfret EA. Surgical resection and liver transplantation for hepatocellular carcinoma. Clin Liver Dis 2015;19:381-99. [Crossref] [PubMed]
- Lin Q, Huang X, Zhong C, et al. Improved survival with radiotherapy in hepatocellular carcinoma with major vascular invasion: A propensity-matched analysis of Surveillance, Epidemiology, and End Results database. Cancer Med 2019;8:515-26. [Crossref] [PubMed]
- Lee S, Kim H, Ji Y, et al. Evaluation of Hepatic Toxicity after Repeated Stereotactic Body Radiation Therapy for Recurrent Hepatocellular Carcinoma using Deformable Image Registration. Sci Rep 2018;8:16224. [Crossref] [PubMed]
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-62. [Crossref] [PubMed]
- Hamaoka M, Kobayashi T, Kuroda S, et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: A retrospective cohort study. Int J Surg 2017;44:223-8. [Crossref] [PubMed]
- Liu S, Guo L, Li H, et al. Postoperative Adjuvant Trans-Arterial Chemoembolization for Patients with Hepatocellular Carcinoma and Portal Vein Tumor Thrombus. Ann Surg Oncol 2018;25:2098-104. [Crossref] [PubMed]
- Zhang X, Wang K, Wang M, et al. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget 2017;8:29416-27. [Crossref] [PubMed]
- Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594. [Crossref] [PubMed]
- Lei Z, Li J, Wu D, et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg 2016;151:356-63. [Crossref] [PubMed]
- Takenouchi T, Komori O, Eguchi S. An extension of the receiver operating characteristic curve and AUC-optimal classification. Neural Comput 2012;24:2789-824. [Crossref] [PubMed]
- Kim J, Hwang IC. Drawing Guidelines for Receiver Operating Characteristic Curve in Preparation of Manuscripts. J Korean Med Sci 2020;35:e171. [Crossref] [PubMed]
- Coutant C, Olivier C, Lambaudie E, et al. Comparison of models to predict nonsentinel lymph node status in breast cancer patients with metastatic sentinel lymph nodes: a prospective multicenter study. J Clin Oncol 2009;27:2800-8. [Crossref] [PubMed]
- Van Calster B, Wynants L, Verbeek JFM, et al. Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators. Eur Urol 2018;74:796-804. [Crossref] [PubMed]
- Okamura Y, Sugiura T, Ito T, et al. The tumor diameter cut-off for predicting microscopic intrahepatic metastasis of hepatocellular carcinoma patients without treatment history differs from that of hepatocellular carcinoma patients with a treatment history. Clin Transl Oncol 2020;22:319-29. [Crossref] [PubMed]
- Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol 2006;12:7561-7. [Crossref] [PubMed]
- Mähringer-Kunz A, Steinle V, Düber C, et al. Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: The more, the worse? Liver Int 2019;39:324-31. [Crossref] [PubMed]
- Jeong SW, Jang JY, Shim KY, et al. Practical effect of sorafenib monotherapy on advanced hepatocellular carcinoma and portal vein tumor thrombosis. Gut Liver 2013;7:696-703. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Matsuyama Y, et al. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: A Japanese nationwide survey. Hepatology 2017;66:510-7. [Crossref] [PubMed]
- Chen JS, Wang Q, Chen XL, et al. Clinicopathologic characteristics and surgical outcomes of hepatocellular carcinoma with portal vein tumor thrombosis. J Surg Res 2012;175:243-50. [Crossref] [PubMed]
- Sakata J, Shirai Y, Wakai T, et al. Preoperative predictors of vascular invasion in hepatocellular carcinoma. Eur J Surg Oncol 2008;34:900-5. [Crossref] [PubMed]
- Soin AS, Bhangui P, Kataria T, et al. Experience With LDLT in Patients With Hepatocellular Carcinoma and Portal Vein Tumor Thrombosis Postdownstaging. Transplantation 2020;104:2334-45. [Crossref] [PubMed]
- Jonas S, Bechstein WO, Steinmüller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001;33:1080-6. [Crossref] [PubMed]
- Renne SL, Woo HY, Allegra S, et al. Vessels Encapsulating Tumor Clusters (VETC) Is a Powerful Predictor of Aggressive Hepatocellular Carcinoma. Hepatology 2020;71:183-95. [Crossref] [PubMed]
- Wada H, Nagano H, Yamamoto H, et al. Expression pattern of angiogenic factors and prognosis after hepatic resection in hepatocellular carcinoma: importance of angiopoietin-2 and hypoxia-induced factor-1 alpha. Liver Int 2006;26:414-23. [Crossref] [PubMed]
- Xiaohong S, Huikai L, Feng W, et al. Clinical significance of lymph node metastasis in patients undergoing partial hepatectomy for hepatocellular carcinoma. World J Surg 2010;34:1028-33. [Crossref] [PubMed]
- Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:835-53. [Crossref] [PubMed]
- Terentiev AA, Moldogazieva NT. Alpha-fetoprotein: a renaissance. Tumour Biol 2013;34:2075-91. [Crossref] [PubMed]
- Bai DS, Zhang C, Chen P, et al. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep 2017;7:12870. [Crossref] [PubMed]
- Zheng Y, Zhu M, Li M. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma. J Cancer Res Clin Oncol 2020;146:2439-46. [Crossref] [PubMed]
- Zhou L, Rui JA, Wang SB, et al. Risk factors of poor prognosis and portal vein tumor thrombosis after curative resection of solitary hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2013;12:68-73. [Crossref] [PubMed]
- Zhang XP, Chai ZT, Gao YZ, et al. Postoperative adjuvant sorafenib improves survival outcomes in hepatocellular carcinoma patients with microvascular invasion after R0 liver resection: a propensity score matching analysis. HPB (Oxford) 2019;21:1687-96. [Crossref] [PubMed]
- Zhang ZY, Guan J, Wang XP, et al. Outcomes of adolescent and young patients with hepatocellular carcinoma after curative liver resection: a retrospective study. World J Surg Oncol 2022;20:210. [Crossref] [PubMed]
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [Crossref] [PubMed]
- Guarino M, Cucchetti A, Pontillo G, et al. Pattern of macrovascular invasion in hepatocellular carcinoma. Eur J Clin Invest 2021;51:e13542. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Verna EC, Patel YA, Aggarwal A, et al. Liver transplantation for hepatocellular carcinoma: Management after the transplant. Am J Transplant 2020;20:333-47. [Crossref] [PubMed]
- Cerrito L, Annicchiarico BE, Iezzi R, et al. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: Beyond the known frontiers. World J Gastroenterol 2019;25:4360-82. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65:938-43. [Crossref] [PubMed]
- Wang K, Guo WX, Chen MS, et al. Multimodality Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Large-Scale, Multicenter, Propensity Mathching Score Analysis. Medicine (Baltimore) 2016;95:e3015. [Crossref] [PubMed]
- Peng SY, Wang XA, Huang CY, et al. Better surgical treatment method for hepatocellular carcinoma with portal vein tumor thrombus. World J Gastroenterol 2018;24:4527-35. [Crossref] [PubMed]


