
Beauty is in the explainable artificial intelligence (XAI) of the “agnostic” beholder
In the October 2022 issue of Translational Cancer Research, Ladbury et al. focused on model-agnostic explainable artificial intelligence (XAI) frameworks and offered an insightful means of introducing oncology machine learning (ML) models (1). Using such frameworks presents the opportunity to improve the understanding of ML models, which is pivotal for their integration in the clinical environment. They employed a non-systematic review-based approach, and aimed to highlight current applications of XAI agnostic frameworks in oncology-related domains. Their choice of taxonomy helped unveil the benefits of explainability and how this has been applied in the literature.
We surmise future readers will refer to this work as a starting point for using these tools. Applications might be multifold, such as helping with threshold or feature selections and inspiring confidence in the model by quantitative visualizations of how the predictions are calculated. Nevertheless, if the oncology community is the overarching target audience, our oncology piers will find the manuscript format the best fit to explore their XAI applications. As humans, we require explanations. The authors identified several fields in oncology research where explainable ML was broadly applicable, including prognostication, diagnosis, radiomics, and treatment selection, to mention but a few. The potential crosstalk amongst those applications can serve an additional role for XAI with respect to prescriptive or semantic analytics. The former can simulate outcomes for all possible scenarios; the latter can recognize the semantic relationships between data attributes to discover new information and possibly, use XAI to address complex interrogatives. Multi-sourced data integration resulting in “Digital Twin” frameworks for predictive oncology can provide a paradigm shift for precision cancer care and XAI can be perfect for model interpretability (2).
The authors acknowledged that XAI might carry some inherent inaccuracies, but merely some general framework would be unifying for general applications, whilst other “underutilized” frameworks might yield favorable results without needing to turn to more complicated and less interpretable ML models. Alternatively, XAI can potentially be used as an auxiliary tool to improve computing Cox model hazard ratios. This is because XAI remains by nature a qualitative entity that can guide subsequent quantitative analyses not essentially included under the XAI umbrella, such as confirmatory Cox regression. Equally, feature interactions identified by the ML models and supported by the agnostic XAI frameworks can be further validated by conventional statistical tests and replicated in separate datasets.
The XAI concept is modality specific. For instance, in structured data analysis, XAI aims to the identification of those variables influencing the model output remains the primary focus. Instead, in image analysis, the focus drifts to the identification of the regions of interest. Herein, although literature search was not limited to structured data, a large proportion of identified studies comprised structured datasets potentially due to difficulty in creating imaging datasets. There is an increasing need to shape large, composite data derived from multiomic analyses for oncology patients.
In precision medicine, the use of XAI for analyzing individual patients has been convincingly demonstrated (3). Going forward, XAI should apply to any point of patient care. The author’s rationale is that the available studies and patient care disciplines do not necessarily have a uniform flow that would lend itself to such a figure. This is true, as one treatment rarely, if ever, is definitively shown to improve outcomes for all important endpoints (4).
In oncology, where patients’ care, health, and beneficence matter, the reliability and accuracy of information and data quality are critical, which depends entirely on the data source. Closer collaboration between oncologists and computer scientists is required. A golden opportunity is arising for electronic health records to be safely shared between hospitals or institutions to ensure data integration, maintain confidentiality and proper use of the information for reliable choice of cares.
In an era of stringent economics, the agnostic XAI applications can potentially confer economic benefits; that is, by implementing AI forecasting models and XAI frameworks, end-users can explain how different policy scenarios are explored and favor the most effective strategy to enhance oncology economics. Ultimately, there will be some challenges as XAI developers will realize that systems are affected by human interactions and human-like traits. Arguably, no controlling mechanism against the creation of intentionally misleading interpretations exists, which can hide biases (5). Ironically, our team has recently demonstrated the potential influence of human factors on the surgical effort exerted by end-users using agnostic XAI models (6).
Currently, the specific cancer types being in the forefront to receive the benefits from AI-based devices in clinical practice are breast cancer, lung cancer, and prostate cancer, likely as the direct reflection of their higher incidence compared with other cancer types. Scratching the surface of the lower incidence cancers is now becoming a necessity. One such cancer, epithelial ovarian cancer (EOC) is the leading cause of death from gynaecological malignancies in the developed countries (7). The extraordinary diversity of EOC continues to offer clues to the underlying causes but also reinforces the need for a global effort escalation to control the disease. Understanding the EOC trajectory requires a vast amount of information, to be handled by different computational approaches, well beyond the capacity of traditional statistics. We have meticulously evaluated the clinical applications of AI models in the surgical outcomes related to EOC to determine areas of future research (8-10). The application of such AI-empowered approaches in the field of EOC holds the potential of upgrading the approach to patient-oriented decision-making (11). A schematic representation of the proposed application of XAI methods in the surgical management of EOC is illustrated in Figure 1.
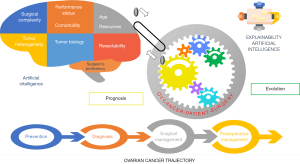
Gynaecological oncology surgeons should soon become up to date with the applications of this modern technology. Nevertheless, further research is warranted to support the agnostic features of explainability and interpretability for predictive and prognostic issues in EOC. Robust, prospective evaluation will be required to secure AI system’s safety and efficacy. That will entail using performance metrics that go beyond measures of technical accuracy (12). We envisage exploring the applicability of XAI methods in the hope to develop a holistic ovarian cancer AI framework and rendering this new technology clinically attractive to oncology specialists. We anticipate that XAI-based clinical EOC research will lead to a paradigm shift in EOC treatment. Integration of patient- and tumor-specific data, in addition to data reflecting surgical capacity and human factors will additionally augment surgical decision support during EOC cytoreductive surgery. It is sensible to expect a multidisciplinary effort to advance the diagnosis, treatment, and prognosis of EOC through the development of not only Explainable but also Collaborative, Adaptive, and Responsible AI solutions. This type of advanced AI solutions has been recently described as Hybrid Human and Artificial Intelligence (13). The hybrid intelligence (HI) solutions should (I) recognize and exploit skill complementarity between humans and machines, prevent human biases (e.g., confirmation bias) and limitations, (II) adapt and learn not only from data but also from experiences and dialogues with human, and (III) act according to legal constrains and moral values.
From this narrative review (1), one could argue that there were no studies predicting certain outcomes that are directly linked to treatment consequences and could therefore be regarded as clinically relevant decision support. Avoiding an overpromising language, XAI frameworks are not to replace clinicians but to equip them with the relevant tools that may improve patient outcomes and time utilization in the clinical workflow. The ultimate key step for clinical translation will be the external data validation to enhance real world clinical relevance. Model-agnostic XAI techniques are specifically designed to serve this purpose; being generally applicable. Indeed, they maintain their flexibility by operating solely on the basis of relating the model input to its output.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2664/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ladbury C, Zarinshenas R, Semwal H, et al. Utilization of model-agnostic explainable artificial intelligence frameworks in oncology: a narrative review. Transl Cancer Res 2022;11:3853-68. [Crossref] [PubMed]
- Barbiero P, Viñas Torné R, Lió P. Graph Representation Forecasting of Patient's Medical Conditions: Toward a Digital Twin. Front Genet 2021;12:652907. [Crossref] [PubMed]
- Kobylińska K, Orłowski T, Adamek M, et al. Explainable Machine Learning for Lung Cancer Screening Models. Applied Sciences 2022;12:1926. [Crossref]
- Chhabra KR, Sacks GD, Dimick JB. Surgical Decision Making: Challenging Dogma and Incorporating Patient Preferences. JAMA 2017;317:357-8. [Crossref] [PubMed]
- Slack D, Hilgard S, Jia E, et al. Fooling lime and shap: Adversarial attacks on post hoc explanation methods. In Proceedings of the AAAI/ACM Conference on AI, Ethics, and Society, 2020:180-6.
- Laios A, Kalampokis E, Johnson R, et al. Factors Predicting Surgical Effort Using Explainable Artificial Intelligence in Advanced Stage Epithelial Ovarian Cancer. Cancers (Basel) 2022;14:3447. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Laios A, Gryparis A, DeJong D, et al. Predicting complete cytoreduction for advanced ovarian cancer patients using nearest-neighbor models. J Ovarian Res 2020;13:117. [Crossref] [PubMed]
- Laios A, Katsenou A, Tan YS, et al. Feature Selection is Critical for 2-Year Prognosis in Advanced Stage High Grade Serous Ovarian Cancer by Using Machine Learning. Cancer Control 2021;28:10732748211044678. [Crossref] [PubMed]
- Laios A, De Oliveira Silva RV, Dantas De Freitas DL, et al. Machine Learning-Based Risk Prediction of Critical Care Unit Admission for Advanced Stage High Grade Serous Ovarian Cancer Patients Undergoing Cytoreductive Surgery: The Leeds-Natal Score. J Clin Med 2021;11:87. [Crossref] [PubMed]
- Wagner M, Schulze A, Haselbeck-Köbler M, et al. Artificial intelligence for decision support in surgical oncology - a systematic review. Art Int Surg 2022;2:159-72. [Crossref]
- Kelly CJ, Karthikesalingam A, Suleyman M, et al. Key challenges for delivering clinical impact with artificial intelligence. BMC Med 2019;17:195. [Crossref] [PubMed]
- Akata Z, Balliet D, de Rijke M, et al. A Research Agenda for Hybrid Intelligence: Augmenting Human Intellect with Collaborative, Adaptive, Responsible, and Explainable Artificial Intelligence. Computer 2020;53:18-28. [Crossref]


