
Leukoencephalopathy in children with acute lymphoblastic leukemia after chemotherapy: a retrospective monocenter study
Highlight box
Key findings
• Chemotherapy is an important risk factor related to leukoencephalopathy. Radiological features and laboratory results may help the identification of leukoencephalopathy.
What is known and what is new?
• The main risk factors of LE include chemotherapy drugs, immunosuppressants and hypertension. The occurrence of LE leads to a series of structural and functional sequelae of the nervous system in long term.
• Radiological features and abnormal laboratory results help to identify leukoencephalopathy in early stage and can improve brain development in the long term.
What is the implication, and what should change now?
• To identify the LE in early stage of ALL patients after chemotherapy with radiological findings and laboratory results will improve the prognosis and prevent serious neurological sequelae. Doctors should pay more attention to these findings and try to find more clues of the occurrence of LE to help the patients.
Introduction
Acute lymphoblastic leukemia (ALL) is one of the most common cancers in children. Leukoencephalopathy (LE), which is recognized as a neurological complication of ALL, affects the long-term prognosis and presents with a series of structural and functional sequelae of the nervous system (1-3). LE is caused by the defects in the integrity of myelin sheath (1), usually happens after chemotherapy. The most prominent feature of LE is the change in white and gray matter volume, with the reduction of white matter being the most obvious (4,5).
Posterior reversible encephalopathy syndrome (PRES), a type of LE, is reported mostly related to neoplastic and renal disorders, while its main risk factors include chemotherapy drugs, immunosuppressants, and hypertension due to recent studies (6-8). Thus, metabolic and microvascular changes potentially play an important role in the development of LE (9).
Antineoplastic therapy will increase the risk of LE (10-12). During ALL therapy, the concentrations of glutathione and oxidized glutathione increase significantly while the ratio of which decreases, suggesting that the extracellular environment becomes oxidized and early apoptotic events occur (13).
Chemotherapy has been affirmed to strongly associated to LE, thus the risk for increasing exposure to chemotherapy to cause LE is high (14-16). To determine the effects of chemotherapy in ALL children, researchers compared 24 survivors treated with chemotherapy for 3–11 years to 21 controls (17). The results indicated that the brain volumes of survivors were definitely reduced in certain regions, including the white matter in the frontal and parietal lobes, the gray matter in the occipital lobe, and both the white and gray matters in subcortical area. Further, the fractional amplitude of low-frequency fluctuation (fALFF) values for the survivor group showed an obvious decrease in the left lingual gyri, calcarine gyri, and left mid frontal gyrus, while the fALFF values in the matched control group were higher in the corresponding regions, indicating the potential damage from chemotherapy (18). Using tract-based spatial statistics, fractional anisotropy and axial diffusivity were found to decrease in several white matter regions, and the mean diffusivity and radial diffusivity were higher in patients treated with chemotherapy (19). Measurements of the cerebrospinal fluid biomarkers of brain injury among ALL children revealed that increased glial fibrillary acidic protein (RR, 1.23; 95% CI: 1.09–1.40), myelin basic protein (RR, 1.06; 95% CI: 1.01–1.11), and total tau (RR, 1.76; 95% CI: 1.11–2.78) increased the risk of LE 5 years after diagnosis (P<0.001) (20).
The study is to investigate the clinical and neuroimaging features of LE in childhood ALL patients, thus help the doctors to arouse the awareness of the disease and to improve prognosis. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2180/rc).
Methods
In this retrospective study, we analyzed the clinical data of childhood ALL patients to develop LE after chemotherapy with the control of those without LE who also accepted ALL chemotherapy.
Study population
17 pediatric patients with ALL who developed LE between May 2011 and April 2021, and 17 controls with ALL without LE treated at the Children’s Hospital of Soochow University were enrolled in this study as experimental group. Relative clinical data including general information, routine blood tests, blood biochemical examinations, coagulation function, routine tests, biochemistry indicators in the cerebrospinal fluid, and imaging examinations were collected.
Based on correlative references (1,10,14), the criteria for the diagnosis of ALL complicated LE were: the clinical manifestations of ALL preceded the symptoms of LE, and the patients presented with clinical symptoms of acute brain dysfunction such as seizures, headache, nausea, vomiting, paralysis of limbs or faces, paresthesia, aphasia, ataxia, and altered consciousness. On cranial magnetic resonance imaging (MRI), the lesion was confirmed to accord with the characteristics of LE. First, the lesions were mainly distributed in the white matter areas of the parietal lobe, frontal lobes, occipital lobe, periventricular area, cerebellum, and brainstem. Second, abnormal signals could also appear in gray matter or gray matter nuclei. Third, the lesion was isointense or slightly hypointense on T1WI and hyperintense on T2WI or fluid-attenuated inversion recovery (FLAIR).
Inclusion criteria: Enrolled patients were diagnosed with B-cell ALL (B-ALL) based on the clinical manifestations of the disease and auxiliary examinations. All patients were treated with a single chemotherapy-only protocol, and never received radiotherapy, transplantation, chimeric antigen receptor T cell (CAR-T), or other treatment before enrollment or during follow-up. The experimental group should meet the above diagnostic criteria. The control group were matched by sex, age at diagnosis of ALL, and duration of ALL treatment.
Patients with the following conditions were excluded: central nervous system leukemia (diagnosed by detection of leukemic cells in the cerebrospinal fluid after lumbar puncture), intracranial infection, cerebrovascular disease (cerebral hemorrhage or cerebral infarction), intracranial mass, and other brain damage.
Statistical analysis
The SAS 9.4 (SAS Institute Inc., Cary, NC) software package was used for data analysis. For quantitative variables, data with a normal distribution were presented as the arithmetic mean and standard deviation and analyzed with the T test or were presented as the median and quartile and examined with the Wilcoxon test. For categorical variables, Fisher’s exact test was adopted. P<0.05 indicated the difference had statistical significance.
Ethics and consent
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the Children’s Hospital of Soochow University (No.2021CS093) and individual consent for this retrospective analysis was waived.
Results
Demographic data
Table 1 presents the demographic characteristics of the experimental and control groups. Among the 17 patients, 12 were boys and 5 were girls. Patients were diagnosed with ALL between the ages of 2.04 and 12.87. The majority of patients (88.24%) were classified as low-risk and moderate-risk. LE occurred 0.02–3.18 years after diagnosis, which happened at their ages of 2.36–13.05 years old. Patients had received 0–26 times of intrathecal chemotherapy (a triple therapy of methotrexate, adriamycin and dexamethasone) before the onset of LE, and 1–7 types of intravenous chemo-drugs within 4 weeks before the onset of LE.
Table 1
| Variable | LE (n=17) | Control (n=17) | W/ t/ χ2 | P |
|---|---|---|---|---|
| Sex, n (%) | 0.0000 | 1.0000 | ||
| Male | 12 (70.59) | 12 (70.59) | ||
| Female | 5 (29.41) | 5 (29.41) | ||
| Age at diagnosis of ALL, years | 7.12±3.20 | 6.71±3.47 | 0.36 | 0.7237 |
| Age at diagnosis of LE, years | 7.85±3.25 | 7.39±3.57 | 0.39 | 0.6993 |
| Duration of ALL treatment, years | 0.59 [0.32, 0.79] | 0.47 [0.15, 0.75] | 285.0000 | 0.6815 |
| Treatment risk stratum | 0.1357 | 1.0000 | ||
| Low | 9 (52.94) | 8 (47.06) | ||
| Moderate | 6 (35.29) | 7 (41.18) | ||
| High | 2 (11.76) | 2 (11.76) | ||
| Main chemotherapy drugs within 4 weeks before onset of LE | ||||
| Methotrexate | 9 (52.94) | 7 (41.18) | 0.4722 | 0.7319 |
| Asparaginase | 6 (35.29) | 8 (47.06) | 0.4857 | 0.7283 |
| Daunorubicin | 5 (29.41) | 5 (29.41) | 0.0000 | 1.0000 |
| Vincristine | 4 (23.53) | 9 (52.94) | 3.1136 | 0.1571 |
| Cyclophosphamide | 2 (11.76) | 4 (23.53) | 0.8095 | 0.6562 |
| Cytarabine | 2 (11.76) | 5 (29.41) | 1.6190 | 0.3983 |
| Numbers of IT injection | 10 [6, 14] | 11±6.38 | 265.5000 | 0.9701 |
Data were presented as mean ± SD, n (%), or median [P25, P75] values. ALL, acute lymphocytic leukemia; LE, leukoencephalopathy; IT, intrathecal.
An equal number of controls matched exactly in sex (male: female, both 12:5), age at diagnosis of ALL (6.71±3.47, t=0.36, P=0.7237), and duration of ALL treatment [0.47 (0.15, 0.75), W=285.0000, P=0.6815]. The control group also received only chemotherapy and followed a similar treatment regimen.
Clinical features of LE
Most patients (16/17, 94.12%) presented neurological symptoms in various forms, and the symptoms in 70.59% (12/17) of patients disappeared after treatment. Seizure (7/17, 41.18%) was the most common symptom. There were three cases of focal seizure, three cases of generalized seizure, and one case of focal and generalized seizures in the same patient. The main symptoms of seizure were limb trembling (6/7), locked gaze (5/7), and stiffness (1/7). The second most common symptoms were nausea (5/17, 29.41%), vomiting (5/17, 29.41%), and paralysis (5/17, 29.41%). Paralysis consisted of central facial palsy (3/17, 17.65%), hemiplegia (2/17, 11.76%), and paraplegia (1/17, 5.88%). One patient had both facial palsy and hemiplegia. Among patients who experienced numbness (4/17, 23.53%), three out of four also suffered from paralysis. In addition, numbness occurred in the two limbs (2/17, 11.76%) or all four limbs (2/17, 11.76%). Other symptoms include slurred speech (4/17, 23.53%), somnolence (3/17, 17.65%), gait problems (3/17, 17.65%), fatigue (2/17, 11.76%), visual impairment (2/17, 11.76%), and headache (1/17, 5.88%). As to visual impairment, one case presented with blurred vision and the other with blindness. Another noteworthy observation was the increase of systolic blood pressure in three patients (P=0.0271, Table 2). None of the controls presented any neurological symptom.
Table 2
| Variable | LE (n=17) | Control (n=17) | W/t | P | Reference values |
|---|---|---|---|---|---|
| CRP (mg/L) | 11.73 [2.75, 20.69] | 0.34 [0.07, 1.73] | 237.0000 | 0.0124* | 0.00–8.00 |
| Na+ (mmol/L) | 130.0 [126.0, 136.0] | 140.0 [136.5, 141.0] | 77.0000 | 0.0001** | 135.0–145.0 |
| K+ (mmol/L) | 3.5±0.9 | 3.7±0.3 | −0.94 | 0.3656 | 3.5–5.5 |
| Ca2+ (mmol/L) | 1.04 [1.00, 1.11] | 1.09 [1.07, 1.20] | 143.0000 | 0.1053 | 1.12–1.23 |
| SBP (mmHg) | 112±16 | 99±12 | 2.33 | 0.0271* | 90–120 |
| DBP (mmHg) | 72 [60, 82] | 65±7 | 179.0000 | 0.3503 | 60–90 |
Data are mean ± SD or median [P25, P75] values. *, P<0.05; **, P≤0.001. LE, leukoencephalopathy; CRP, C-reactive protein; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Laboratory results
Laboratory tests were performed within 1 week prior to abnormal imaging signals. All patients had no fewer than two abnormalities in red blood cells (RBCs), white blood cells (WBCs), and platelets (PLTs). Low hemoglobin (Hb) was found in 15 patients (88.24%), with mild anemia in six cases, moderate anemia in eight cases, and severe anemia in one case. Five patients (29.41%) had coagulant function abnormality, and electrolyte disorder was observed in eight patients (47.06%). Additionally, liver dysfunction occurred in 12 patients (70.59%), but no abnormality was found during renal function examination. Finally, there were three patients with abnormalities in routine tests and in the biochemistry indicators of the cerebrospinal fluid. The details are listed in Table 2.
Table 2 also presents a comparison of some laboratory results of the experimental group to those of the control group. Compared to the matched control group, PLT (P=0.0421), Hb (P=0.0273), creatinine (P=0.0458), and serum sodium (P=0.0001) were markedly lower in patients with LE. Moreover, the LE group had higher levels of CRP (P=0.0124) and ALT (P=0.0108). There were no statistical differences in RBCs, WBCs, and coagulant function.
Imaging characteristics of LE
All patients with LE underwent head MRI examination. Intracranial lesions were mostly extensive, mainly involving the white matter and subcortical white matter regions. However, gray matter and gray matter nuclei were also involved in some cases. On MRI scans, abnormal signals consisted of equal or slightly lower signal on the T1WI sequence, and high signal on the T2WI and FLAIR sequences. Multiple and irregular lesions were observed, distributed in small or large patches (Figures 1-3), mainly in the periventricular area (9/17, 52.94%), parietal lobe (6/17, 35.29%), basal ganglia (5/17, 29.41%), frontal lobes (3/17, 17.65%), occipital lobe (2/17, 11.76%), and thalamus (2/17, 11.76%). Abnormal signal shadows were also seen in the temporal lobe, pons, hippocampus, and corpus callosum. Six children in the LE group had mild or moderate widening of the sulci, and two developed brain atrophy, compared to two children with wider sulci in the control group (P=0.2245).
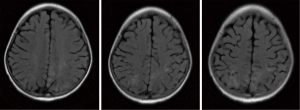
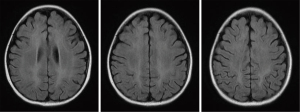
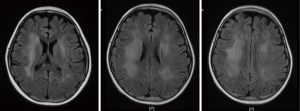
Treatment and prognosis
Chemotherapy was suspended after LE development for all patients undergoing chemotherapy. Eleven children were treated with glucocorticoid pulse therapy: methylprednisolone 15–20 mg/kg/d was given intravenously (the maximum single dose was 1,000 mg/d). After 3 days of continuous administration, the dosage was halved, and oral prednisolone 1–2 mg/kg/d was given sequentially and gradually stopped. The total course of treatment was 1–2 months. Gamma globulin was added (total 2 g/kg over 3 to 5 days) combined with neurotrophic drugs, such as Oxiracetam, B vitamins, and monosialotetrahexosylganglioside sodium. Symptomatic treatment was performed simultaneously to control related symptoms; for example, Mannitol or Glycerol Fructose was used to reduce cranial pressure. Additionally, six patients with mild symptoms were only treated with intravenous gamma globulin and/or neurotrophic drugs.
The overall prognosis of LE was good. The neurological symptoms of 12 patients (70.59%) disappeared completely or improved significantly upon discharge. After treatment, two patients (11.76%) still had seizures and one patient (5.88%) still had headache. Two patients died due to complications from leukemia, none of them from LE. Since both patients with visual impairment died, the prognosis for visual disturbances was uncertain. After discharge, 13 children underwent imaging follow-up. The imaging results showed a significant decrease (7/13, 53.85%) or disappearance (4/13, 30.77%) of abnormal signals within 1 year (Figures 4-6).
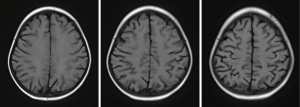
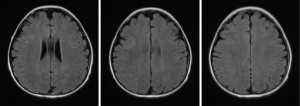
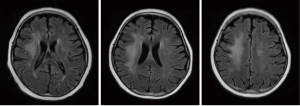
Discussion
Recently, there are increasingly remarkable results achieved in ALL treatment (21,22), but the understanding of central nervous system complications during ALL treatment remains limited. LE, one of the neurological complications of ALL, will strongly affect the cognitive development in survivors (1,23,24). With the aim of detecting the clinical and neuroimaging characteristics of LE, 17 B-ALL children who also suffered from LE were evaluated in this study. The patients were treated with a single chemotherapy-only protocol, and never given radiotherapy, transplantation, CAR-T, or other treatment before or during follow-up. The duration of chemotherapy at the diagnosis of LE varied, with a median of 0.59 years.
In total, 94.14% of patients had neurological symptoms, mostly seizures (7/17, 41.18%), nausea (5/17, 29.41%), vomiting (5/17, 29.41%), paralysis (5/17, 29.41%), and numbness (4/17, 23.53%). We also found that blood pressure was remarkably elevated in patients with LE (P=0.0271). Some symptoms were also reported from previous researches (10,25,26), such as headache (10/11), seizures (7/11), hypertension (4/11), and visual impairment (6/11, 4/13), but consciousness disorder (5/11, 2/13) and vasogenic edema were not detected in our research. Differences in the type or frequency of the symptoms may be related to the different sub-classifications of LE, but it is hard to further classify because of the lack of relevant guides.
In terms of the laboratory results, we found several serum indicators different between experimental and control group. Although it can be ascribed to small sample capacity and coincidence, some logical guesses still deserve further discussion. First of all, we found patients with LE had an obviously higher level of CRP (median=11.73, range, 0.03–54.71) than the controls (median=0.34, range, 0.01–82.38, P=0.0124). As CRP has been affirmed a strong relationship with inflammation, which happens to be one of the definite etiologies of demyelination (27) and may be implicated in the mechanisms of LE (9), a higher level of serum CRP may increase the risk of LE via acute or chronic neuroinflammation. The innate and adaptive immune systems are believed to affect the development of and recovery from demyelinating diseases (28). Recently, mast cells and innate lymphoid cells were shown to exert profound effects on central nervous system inflammatory diseases (29). But due to the lack of related research, this field remains riddled with mystery.
Secondly, the ion channels, especially calcium and sodium channels have been reported to intensively connected with the regeneration and apoptosis of myelin sheath (30,31). We also found a significant difference (P=0.0001) of serum sodium level between LE and control group, but failed to observe the same in serum calcium, which may be concluded to the limited sample capacity. Also, no statistical difference of serum potassium between the two groups was found in our study.
Hypertension has been confirmed to be the key risk factor of PRES, a subtype of LE due to previous studies (32,33). In this research, we also found blood pressure related to the occurrence of LE, especially systolic blood pressure (P=0.0271). The administration of steroid may be responsible for the result: it is difficult to keep a perfect balance between protecting neural cells from methotrexate (MTX) and raising blood pressure in clinical practice.
From the imaging examination results, the lesions were mainly concentrated in the periventricular area (9/17, 52.94%), parietal lobe (6/17, 35.29%), and basal ganglia (5/17, 29.41%), presented multiple and irregular, distributed in small or large patches. The location and morphology of the lesions were similar to previous studies on PRES (7,10,26), but the exact incidence in different brain regions remains unknown. Although six children showed wider sulci in the LE group, there is no statistical difference between children with and without LE (P=0.2245). The change of sulci can also be seen in some cases during follow-up, but it is hard to determine whether it is the result of corticosteroid treatment or the progression of ALL.
Most of the symptoms (12/17, 70.59%) improved or disappeared quickly after active treatment. During the follow-up period, 84.62% (11/13) of the children showed significant improvements in the head MRI review within 1 year. According to previous studies, few interventions can reverse LE except PRES (1,7,26). In our study, just five patients were diagnosed with PRES, so the effect of aggressive treatment was significant.
Although it is difficult to measure the neurotoxic effects of every chemotherapy drug, MTX has a tendency to be neurotoxic. With higher serum concentrations of MTX, patients were more likely to demonstrate executive dysfunction (P<0.02) (34-36). Changes could also be seen in the cortices of the dorsolateral prefrontal region and white matter microstructures of the frontal striatum (36), with a decrease in the efficiency of the neural network (2). Based on the resulting electroencephalogram data, ALL children had lower amplitude in the P1 component (sensory processing) and mismatches in the negativity component (working memory), P3a component (attentional orienting), and P3b component (target detection) (37). In summary, chemotherapy drugs, MTX in particular, have an important effect on the occurrence of LE in ALL children.
However, we didn’t find the numbers of IT injection statistically different between the experimental and control group (P=0.9701) in our study. This may due to the protection of dexamethasone given together with IT-MTX and folic acid administrated afterwards (38), to verify which a larger sample research is necessary in the future.
Conclusions
In conclusion, the pathogenesis of ALL combined with LE is complex and the clinical symptoms are diverse. With regard to the pathogenesis, chemotherapy is a major risk factor, and immunity dysfunction may also play an important role. As for the clinical features, the main symptoms include seizure, nausea, vomiting, paralysis, and numbness, and neuroradiological lesions are mostly distributed in the periventricular area, parietal lobe, and basal ganglia. In particular, when a child with ALL presents with neurological symptoms accompanied by multiple laboratory abnormalities, the doctor should carefully investigate the presence of LE. Early detection and timely treatment can improve the brain development of children with LE.
Acknowledgments
Funding: This work was supported by the Technology Project of the Health Committee of Jiangsu Province (No. H2018010), and Suzhou Science and Technology Bureau and Suzhou Health and Family Planning Commission (No. KJXW2019023).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2180/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2180/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2180/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the Children’s Hospital of Soochow University (No.2021CS093) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Partap S, Russo S, Esfahani B, et al. A Review of Chronic Leukoencephalopathy among Survivors of Childhood Cancer. Pediatr Neurol 2019;101:2-10. [Crossref] [PubMed]
- Kesler SR, Ogg R, Reddick WE, et al. Brain Network Connectivity and Executive Function in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Brain Connect 2018;8:333-42. [Crossref] [PubMed]
- Sabin ND, Cheung YT, Reddick WE, et al. The Impact of Persistent Leukoencephalopathy on Brain White Matter Microstructure in Long-Term Survivors of Acute Lymphoblastic Leukemia Treated with Chemotherapy Only. AJNR Am J Neuroradiol 2018;39:1919-25. [Crossref] [PubMed]
- Zhou C, Zhuang Y, Lin X, et al. Changes in neurocognitive function and central nervous system structure in childhood acute lymphoblastic leukaemia survivors after treatment: a meta-analysis. Br J Haematol 2020;188:945-61. [Crossref] [PubMed]
- van der Plas E, Spencer Noakes TL, Butcher DT, et al. Quantitative MRI outcomes in child and adolescent leukemia survivors: Evidence for global alterations in gray and white matter. Neuroimage Clin 2020;28:102428. [Crossref] [PubMed]
- Darwish AH. Posterior Reversible Encephalopathy Syndrome in Children: A Prospective Follow-up Study. J Child Neurol 2020;35:55-62. [Crossref] [PubMed]
- Danhofer P, Tomečková M, Černá D, et al. Prognostic factors and seizure outcome in posterior reversible encephalopathy syndrome (PRES) in children with hematological malignancies and bone marrow failure: A retrospective monocentric study. Seizure 2019;72:1-10. [Crossref] [PubMed]
- Pirola JP, Baenas DF, Haye Salinas MJ, et al. Posterior reversible leukoencephalopathy syndrome: Case series and review of the literature. Reumatol Clin 2020;16:169-73. (Engl Ed). [Crossref] [PubMed]
- Wang M, Norman JE, Srinivasan VJ, et al. Metabolic, inflammatory, and microvascular determinants of white matter disease and cognitive decline. Am J Neurodegener Dis 2016;5:171-7. [PubMed]
- Tang JH, Tian JM, Sheng M, et al. Study of Posterior Reversible Encephalopathy Syndrome in Children With Acute Lymphoblastic Leukemia After Induction Chemotherapy. J Child Neurol 2016;31:279-84. [Crossref] [PubMed]
- Shalabi H, Wolters PL, Martin S, et al. Systematic Evaluation of Neurotoxicity in Children and Young Adults Undergoing CD22 Chimeric Antigen Receptor T-Cell Therapy. J Immunother 2018;41:350-8. [Crossref] [PubMed]
- Zając-Spychała O, Pawlak MA, Karmelita-Katulska K, et al. Long-term brain status and cognitive impairment in children treated for high-risk acute lymphoblastic leukemia with and without allogeneic hematopoietic stem cell transplantation: A single-center study. Pediatr Blood Cancer 2020;67:e28224. [Crossref] [PubMed]
- Moore IMK, Koerner KM, Gundy PM, et al. Changes in Oxidant Defense, Apoptosis, and Cognitive Abilities During Treatment for Childhood Leukemia. Biol Res Nurs 2018;20:393-402. [Crossref] [PubMed]
- Cheung YT, Sabin ND, Reddick WE, et al. Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy: a longitudinal analysis. Lancet Haematol 2016;3:e456-66. [Crossref] [PubMed]
- Zając-Spychała O, Pawlak MA, Karmelita-Katulska K, et al. Long-term brain structural magnetic resonance imaging and cognitive functioning in children treated for acute lymphoblastic leukemia with high-dose methotrexate chemotherapy alone or combined with CNS radiotherapy at reduced total dose to 12 Gy. Neuroradiology 2017;59:147-56. [Crossref] [PubMed]
- Kesler SR, Gugel M, Huston-Warren E, et al. Atypical Structural Connectome Organization and Cognitive Impairment in Young Survivors of Acute Lymphoblastic Leukemia. Brain Connect 2016;6:273-82. [Crossref] [PubMed]
- van der Plas E, Schachar RJ, Hitzler J, et al. Brain structure, working memory and response inhibition in childhood leukemia survivors. Brain Behav 2016;7:e00621. [Crossref] [PubMed]
- Zou D, Wen F, Zeng H, et al. Improving brain function of pediatric acute lymphoblastic leukemia patients after induction chemotherapy, a pilot self-contrast study by fractional amplitude of low-frequency fluctuation. J Clin Neurosci 2019;66:149-55. [Crossref] [PubMed]
- Zou L, Su L, Xu J, et al. Structural brain alteration in survivors of acute lymphoblastic leukemia with chemotherapy treatment: A voxel-based morphometry and diffusion tensor imaging study. Brain Res 2017;1658:68-72. [Crossref] [PubMed]
- Cheung YT, Khan RB, Liu W, et al. Association of Cerebrospinal Fluid Biomarkers of Central Nervous System Injury With Neurocognitive and Brain Imaging Outcomes in Children Receiving Chemotherapy for Acute Lymphoblastic Leukemia. JAMA Oncol 2018;4:e180089. [Crossref] [PubMed]
- Rafei H, Kantarjian HM, Jabbour EJ. Recent advances in the treatment of acute lymphoblastic leukemia. Leuk Lymphoma 2019;60:2606-21. [Crossref] [PubMed]
- Pui CH. Precision medicine in acute lymphoblastic leukemia. Front Med 2020;14:689-700. [Crossref] [PubMed]
- Tamnes CK, Zeller B, Amlien IK, et al. Cortical surface area and thickness in adult survivors of pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer 2015;62:1027-34. [Crossref] [PubMed]
- Darling SJ, De Luca C, Anderson V, et al. White Matter Microstructure and Information Processing at the Completion of Chemotherapy-Only Treatment for Pediatric Acute Lymphoblastic Leukemia. Dev Neuropsychol 2018;43:385-402. [Crossref] [PubMed]
- Eryılmaz MK, Mutlu H, Salim DK, et al. Fatal posterior revesible leukoencephalopathy syndrome associated coma induced by bevacizumab in metastatic colorectal cancer and review of literature. J Oncol Pharm Pract 2016;22:806-10. [Crossref] [PubMed]
- Lin W, Xie J, Zhang J, et al. Posterior reversible encephalopathy syndrome in children with acute lymphoblastic leukemia during remission induction chemotherapy: a single-center retrospective study. Minerva Pediatr 2019; Epub ahead of print. [Crossref] [PubMed]
- Ohno N, Ikenaka K. Axonal and neuronal degeneration in myelin diseases. Neurosci Res 2019;139:48-57. [Crossref] [PubMed]
- Mayo L, Quintana FJ, Weiner HL. The innate immune system in demyelinating disease. Immunol Rev 2012;248:170-87. [Crossref] [PubMed]
- Brown MA, Weinberg RB. Mast Cells and Innate Lymphoid Cells: Underappreciated Players in CNS Autoimmune Demyelinating Disease. Front Immunol 2018;9:514. [Crossref] [PubMed]
- Song S, Luo L, Sun B, et al. Roles of glial ion transporters in brain diseases. Glia 2020;68:472-94. [Crossref] [PubMed]
- Boscia F, de Rosa V, Cammarota M, et al. The Na(+)/Ca(2+) exchangers in demyelinating diseases. Cell Calcium 2020;85:102130. [Crossref] [PubMed]
- Musioł K, Waz S, Boroń M, et al. PRES in the course of hemato-oncological treatment in children. Childs Nerv Syst 2018;34:691-9. [Crossref] [PubMed]
- Morris EB, Laningham FH, Sandlund JT, et al. Posterior reversible encephalopathy syndrome in children with cancer. Pediatr Blood Cancer 2007;48:152-9. [Crossref] [PubMed]
- Fellah S, Cheung YT, Scoggins MA, et al. Brain Activity Associated With Attention Deficits Following Chemotherapy for Childhood Acute Lymphoblastic Leukemia. J Natl Cancer Inst 2019;111:201-9. [Crossref] [PubMed]
- Jacola LM, Edelstein K, Liu W, et al. Cognitive, behaviour, and academic functioning in adolescent and young adult survivors of childhood acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study. Lancet Psychiatry 2016;3:965-72. [Crossref] [PubMed]
- Krull KR, Cheung YT, Liu W, et al. Chemotherapy Pharmacodynamics and Neuroimaging and Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. J Clin Oncol 2016;34:2644-53. [Crossref] [PubMed]
- Brace KM, Lee WW, Cole PD, et al. Childhood leukemia survivors exhibit deficiencies in sensory and cognitive processes, as reflected by event-related brain potentials after completion of curative chemotherapy: A preliminary investigation. J Clin Exp Neuropsychol 2019;41:814-31. [Crossref] [PubMed]
- Cohen IJ. Defining the appropriate dosage of folinic acid after high-dose methotrexate for childhood acute lymphatic leukemia that will prevent neurotoxicity without rescuing malignant cells in the central nervous system. J Pediatr Hematol Oncol 2004;26:156-63. [Crossref] [PubMed]


