
Problems and countermeasures for surgical resection of primary pulmonary artery intimal sarcoma
Highlight box
Key findings
• The distance between the location of the pulmonary artery intimal sarcoma (PAIS) on computed tomography and the dissection line during surgery needed to be at least 20 mm.
• The median postoperative recurrence period was as short as 10 months.
What is known and what is new?
• It is known that complete resection of PAIS by surgery has a good prognosis, but there are no papers aiming to predict complete resection using preoperative chest CT.
• This is the first study to measure the distance from the tumor to the dissection margin using preoperative contrast-enhanced CT.
What is the implication, and what should change now?
• Intensive care for intrathoracic recurrence follow-up is required for 1 year after surgery even in patients with complete resection pathologically.
Introduction
Pulmonary artery intimal sarcoma (PAIS) is a very rare and poorly understood tumor that arises from the intimal layer of the pulmonary artery (PA). In 1923, Mandelstamm published the first description of this sarcoma (1). Since then, nearly 300 cases have been reported (2-4). The age of onset of PAIS ranges from 13–86 years (5,6), with the majority of cases occurring in the middle-aged generation. The results of chemotherapy or radiotherapy management alone for PAIS are suboptimal. Krüger et al. reported that the median survival time without surgical resection is 1.5 months, whereas it is 10 months with surgery (7), and surgery is still considered necessary as a treatment policy. Interventions reported for PAIS include palliative stenting, total pneumonectomy, and endarterectomy, with or without pneumonectomy, and with or without PA reconstruction. Blackmon et al. reported that the 5-year survival rate was 49.2% for completely resected cases and 0% for incompletely resected cases (8). In other words, since complete resection contributes to prognosis, reconstruction using artificial blood vessels is performed. However, since PAIS is a tumor that grows while replacing the existing vascular endothelium, preoperative diagnosis does not indicate the extent to which the tumor has grown. Therefore, there are few positive resection reports that achieve complete resection. This study reviewed the management of 10 PAIS patients at our institution, assessed short- and long-term outcomes, and assessed the likelihood of complete resection based on preoperative computed tomography (CT), positron emission tomography (PET)-CT, and postoperative pathological results. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1945/rc).
Methods
Study population
We retrospectively reviewed the clinical records of all patients who could be identified through a computer-assisted search of all medical records with a pathological diagnosis of PAIS, primarily diagnosed at the Juntendo University Hospital. From January 2007 to December 2020, 10 patients underwent surgery for PAIS at our institution. The operations were performed with curative intent in all 10 patients. The medical records were retrospectively reviewed to evaluate the clinical characteristics, operable findings, postoperative courses, and long-term results. They all underwent contrast-enhanced CT before surgery. Case No. 1 was operated on in 2007; therefore, a thin-section CT scan was not performed. The remaining nine patients underwent a thin-section CT. The follow-up comprised a combination of outpatient visits and telephone calls. We defined early outcomes as pre-discharge and long-term outcomes as post-discharge. Overall survival was defined as the interval between the date of the operation and the date of death from any cause or the date of the last follow-up (March 1, 2022).
We excluded the following patients from this study: (I) patients with lung parenchymal sarcomas; (II) patients with metastatic sarcomas of the pulmonary vessels and (III) patients with inoperable primary PAISs in a fully palliative situation. This retrospective study was approved by the ethics committee of Juntendo University Hospital (No. 2020115), and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent for this retrospective research was waived.
Surgical techniques
The operations were performed via thoracotomy and median sternotomy in three and seven patients, respectively. The diagnosis of PAIS was histologically proven by frozen section biopsy during the operation. After confirmation of PAIS, the tumor was radically resected, and effort was made to completely remove the tumor. Seven patients underwent surgical resection with hypothermic cardiopulmonary bypass. Nine patients underwent pulmonary resection, including pneumonectomy in eight patients and bilobectomy in one patient. As a surgical policy of our hospital, the operation of the heart and lung was divided into two stages when PA replacement was necessary.
Radiologic evaluations of thin-section computed tomography scan
The findings of preoperative contrast thin-section CT scans were reviewed by the author (HI) and the tumor size was determined preoperatively based on these findings. The size of the solid component was recorded as the maximum diameter in a single axial plane in the mediastinal window condition without a sharpening filter. In addition, all tumors were subsequently evaluated to estimate the tumor size using thin-section CT scans with a 2-mm collimator at our institute. We measured the distance from the site where the tumor was in contact with the PA to the surgical dissection line in all cross sections of the horizontal, sagittal, and coronal sections. The measurements for cases 2 and 3 are shown in Figure 1A,1B.
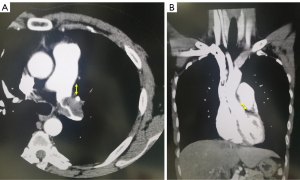
The lung was photographed with a window level of 500–700 H and window depth of 1,000–2,000 H, which was labeled as the “lung window,” and a window level of 30–60 H and window depth of 350–600 H, which was labeled as the “mediastinal window.”
Pathological examination
The hematoxylin and eosin-stained slides of all patients were examined by a pathologist experienced in the field of thoracic tissue tumor pathology. Microscopy and immunohistochemistry for cytokeratins, S-100 protein, desmin, CD34, CD31, Factor VIII and smooth muscle actin were also performed.
We diagnosed PAIS according to the 2021 WHO classification (9). PAIS are malignant mesenchymal neoplasms arising in the large vessels of the pulmonary circulation, which are composed of spindle cells with varying degrees of atypia and variable cellularity.
Statistical analysis
Survival analysis was performed using the Kaplan-Meier method and log-rank test. All data were analyzed using IBM SPSS Statistics (version 23.0; IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
The preoperative patient characteristics are summarized in Table 1. Three (30%) patients were male, and the age at presentation ranged from 26–77 years, with a median age of 61 years. All patients were symptomatic, and the most common symptom was respiratory distress (4/10, 40%). The time from symptom onset to surgery was 1–11 months, with a median of 2.5 months. The range of tumor diameter was 40–92 mm, and the median was 70 mm. PET-CT was performed in four patients with three positive cases (3/4, 75%).
Table 1
| No. | Age (years) | Sex | Symptoms | Time from symptom to surgery (months) | Medical history | Tumor diameter (mm) | PET-CT (SUVmax) |
|---|---|---|---|---|---|---|---|
| 1 | 58 | Female | Dyspnea | 2 | Rheumatoid arthritis | 74 | Not enforced |
| Cervical cancer | |||||||
| 2 | 43 | Male | Chest pain | 11 | Hypertension | 40 | 9.4 |
| 3 | 49 | Female | Fever, cough | 4 | Cervical cancer | 88 | 6.2 |
| 4 | 66 | Female | Cough | 4 | None | 70 | Not enforced |
| 5 | 75 | Female | Hemoptysis | 1 | None | 70 | Not enforced |
| 6 | 69 | Male | Back pain | 2 | Hypertension, type 2 diabetes | 67 | 8.8 |
| 7 | 26 | Female | Dyspnea, back pain | 3 | None | 92 | Not enforced |
| 8 | 77 | Female | Dyspnea | 2 | Hypertension, type 2 diabetes | 40 | Negative |
| 9 | 64 | Female | Dyspnea | 3 | Organized pneumonia | 59 | Not enforced |
| 10 | 33 | Male | Chest pain | 1 | None | 75 | Not enforced |
PET-CT, positron emission tomography-computed tomography; SUVmax, maximum standardized uptake value.
Intraoperative and pathologic results
The surgical operative details are summarized in Table 2. There were three cases in which only lung resection (right middle and lower lobectomy and left pneumonectomy) was completed. However, the final pathological results showed positive findings in all three cases. There were six cases in which PA vascular replacement was required in addition to pulmonary resection. One was a case in which aortic valve replacement was added. The final pathological results showed 3/6 cases had a negative margin. Seven patients had extracorporeal circulation, and the day after, there were three cases in which lung resection was performed in two stages. The median surgical time and blood loss were 363 min (range, 144–595 min) and 515 mL (range, 85–8,100 mL), respectively.
Table 2
| No. | First surgery content | Tumor localization | Extracorporeal circulation | Two-stage surgery | Surgery time (minute) | Blood loss (mL) |
|---|---|---|---|---|---|---|
| 1 | LPN, PAVR, AVR | LPA (C and P), PT, AV | Presence | No | 595 | 440 |
| 2 | LPN | LPA (C and P) | Absence | No | 167 | 120 |
| 3 | RPN, PAVR | RPA (C and P), PT | Presence | Yes | 312+125* | 1,110+235* |
| 4 | RPN, PAVR | RPA (C and P), PT | Presence | Yes | 428+96* | 1,520+40* |
| 5 | RPN, PAVR | RPA (C and P), PT, LPA (C) | Presence | Yes | 296+120* | 2,080+130* |
| 6 | RMLL | RPA (P) | Absence | No | 240 | 85 |
| 7 | PAE | RPA (C), LPA (C) | Presence | No | 240 | 100 |
| 8 | LPN | LPA (C and P) | Absence | No | 144 | 200 |
| 9 | LPN, PAVR | RPA (C), LPA (C and P), PT | Presence | No | 309 | 590 |
| 10 | LPN, PAVR | RPA (C), LPA (C and P), PT | Presence | No | 559 | 8,100 |
*, first surgery + second surgery. LPN, left pneumonectomy; PAVR, pulmonary artery vascular replacement; AVR, aortic valve replacement; LPA, left pulmonary artery; C, center; P, peripheral; PT, pulmonary trunk; AV, aortic valve; RPN, right pneumonectomy; PAE, pulmonary artery endarterectomy; RMLL, right middle and lower lobectomy; RPA, right pulmonary artery.
The CT findings and pathological margin results are summarized in Table 3. In this series, 4/10 cases were observed to have a very small amount of three different morphological components: a leiomyosarcomatous component (No. 1), an osteosarcomatous component (No. 7, 9) and a chondroblastomatous component (No. 8). The positive rate of vascular stumps was 7/10. It was not possible to measure cases in which the surgical procedure was pulmonary endarterectomy (PE) (Case No. 7). Case No. 4 could not be measured because it infiltrated the PAs in the lungs on both sides. All patients with a stump distance of ≤20 mm had a positive stump (6/6). The distance between the tumor and the dissection line during surgery needed to be at least 20 mm (Cases No. 9 and 10). However, although the distance between the tumor and the dissection line during surgery was 25 mm, one case with a positive stump was found (Case No. 6).
Table 3
| No. | Tumor diameter (mm) | Distance between tumor and the dissection line on CT (mm) | Pathological stump results |
|---|---|---|---|
| 1 | 74 | 20 | Negative |
| 2 | 40 | 11 | Positive |
| 3 | 88 | 10 | Positive |
| 4 | 70 | 8 | Positive |
| 5 | 70 | 11 | Positive |
| 6 | 67 | 25 | Positive |
| 7 | 92 | Unmeasurable | Positive |
| 8 | 40 | 6 | Positive |
| 9 | 59 | 25 | Negative |
| 10 | 75 | 23 | Negative |
CT, computed tomography.
Early outcomes
The median lengths of intensive care unit (ICU) and postoperative hospital stays in this series were 4 days (range, 2–13 days) and 15 days (range, 6–134 days), respectively. The most common complication was hoarseness in four patients (median hospitalization was 18.5 days). In these four patients, the median hospitalization of three cases without complications was longer than 7 days. There was one in-hospital death (10%) in the ICU from right heart failure on postoperative day (POD) 12.
Long-term follow-up
The postoperative outcomes are summarized in Table 4. Postoperative recurrence was observed in 8/9 cases, and the median recurrence period was 10 months (range, 3–19 months). All eight patients had intrathoracic recurrence in varying locations: in the lungs (Cases No. 3 and 7), in the PA (Cases No. 2, 4, 6, 9, and 10), and in the superior vena cava (Case No 5). We examined all cases using chest contrast CT to identify postoperative recurrence. One patient (Case No. 6) underwent PET-CT after surgery. It was performed 10 months after the first operation, and the SUVmax was 15.2 due to stump recurrence (tumor size: 34 mm). Postoperative treatment was required in 7/9 cases (operation/chemotherapy/radiotherapy/chemoradiotherapy/heavy ion radiotherapy =1/2/2/1/1). The chemotherapy regimens included cisplatin and doxorubicin in two cases (No. 3 and No. 7) and only paclitaxel in one case because of the patients’ concomitant heart failure (No. 10). The radiation course involved irradiation of the stump of the PA with 2 Gy 25 times. After surgery, two cases used pazopanib. One patient (Case No. 3) did not respond to pazopanib and died from progression of PAIS due to poor performance status 2 months after using pazopanib. Another patient (Case No. 7), who had been using pazopanib for 18 months, still had a stable disease.
Table 4
| No. | ICU days | Hospital stays | Complications | Recurrence [months] | Recurrence site | Postoperative course [months] | Survival [months] | Cause of death |
|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 12 | Right heart failure | No | None | Right heart failure | Dead [0.5] | Perioperative death |
| 2 | 2 | 6 | None | Yes [19] | MPA, RPA | No PT | Dead [21] | Cancer death |
| 3 | 4 | 23 | Hoarseness | Yes [10] | Lung, Liver Kidney, Bone | RT (2 Gy ×25) [3], ChT [10], Pazopanib [11] | Dead [13] | Cancer death |
| 4 | 13 | 33 | Difficulty sputum, PAF | Yes [3] | MPA, RPA | CRT [3] | Dead [8] | Cancer death |
| 5 | 4 | 16 | Hoarseness | Yes [8] | SVC, RA | No PT | Dead [8] | Cancer death |
| 6 | 2 | 7 | None | Yes [5] | PA stump | Operation [11], operation [19] | Dead [24] | Cancer death |
| 7 | 5 | 12 | None | Yes [10] | RUL | ChT [3], operation [9], Pazopanib [18] | Alive [36] | |
| 8 | 3 | 134 | Takotsubo cardiomyopathy, postoperative myastenic crisis | No | None | RT (2 Gy ×25) [3] | Alive [24] | |
| 9 | 3 | 21 | Hoarseness | Yes [18] | PA stump | HIR [18] | Alive [22] | |
| 10 | 5 | 15 | Hoarseness | Yes [9] | MPA, RPA | ChT [9], RT [3 Gy ×15] [10] | Alive [15] |
ICU, intensive care unit; MPA, main pulmonary artery; RPA, right pulmonary artery; PT, postoperative therapy; RT, radiotherapy; ChT, chemotherapy; PAF, paroxysmal atrial fibrillation; SVC, superior vena cava; CRT, chemoradiotherapy; RA, right atrium; PA, pulmonary artery; RUL, right upper lobe; HIR, heavy ion radiotherapy.
The median survival was 15 months (range, 0.5–36 months). The mean recurrence times for margin-positive and margin-negative cases were 9.6 months (range, 3–19 months) and 13.5 months (range, 9–18 months), respectively. The mean survival times for margin-positive and margin-negative cases were 19.1 months (range, 8–36 months) and 19.5 months (range, 17–22 months), respectively. All causes of death were sarcoma, except for one case of perioperative death. No patient was lost to follow-up and no patients switched to another hospital.
Discussion
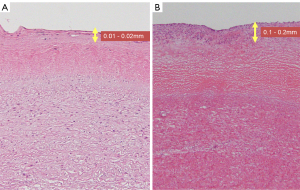
This is the first study to measure the distance from the tumor to the dissection margin using preoperative contrast-enhanced CT. Based on our investigation, the positive rate of vascular stumps was 7/10, and all patients with a stump distance of ≤20 mm had a positive stump (6/6). The distance between the tumor and the dissection line during surgery needed to be at least 20 mm. However, Case No. 6 had a distance of 25 mm with a positive dissected stump. Since PAIS propagates and proliferates in the intima of the PA, it is difficult to determine how far it has progressed by contrast-enhanced CT and PET-CT. In terms of pathological findings, the PAIS extension site is 0.01–0.02 mm for the normal PA intima, whereas the PA intima for PAIS is 0.1–0.2 mm, which is not as thick (Figure 2A,2B). Siordia et al. reported that primary PAIS is better treated with pneumonectomy than with PE, which is better suited for palliative treatment (10). With regard to the pathological findings, it is difficult to make a macroscopic judgment during surgery, and we agree with the opinions of Siordia et al. Extended PAIS emergency surgery is often performed to save lives to remove a tumor that is originally symptomatic and has grown. However, since there are reports of patients living for more than 5 years due to extended surgery (11), one indicator would be that the distance between the tumor and the stump during surgery is 20 mm.
We also reported that cardiovascular and lung surgeries are performed on different days for PAIS surgery. The advantage is that in the case of one-stage surgery, a surgical heart-lung machine tends to cause bleeding, so a two-stage helps ensure a clear surgical field of view. In addition, lung surgery is easier for general thoracic surgeons than surgery with a median sternotomy, by performing a posterior lateral incision. The disadvantage is that it is complicated because the surgery is performed in two stages. For Case No. 10, we attempted to perform the operation in two stages, but on the night of 0 POD of cardiovascular surgery, the bleeding from the trachea did not stop and the operation was accelerated. We believe that two-stage surgery is more effective, for example, when there is a high probability of adhesions in the chest cavity.
Kim et al. reported that early detection contributes significantly to prognosis (12). For every doubling of time from symptom onset to diagnosis, the odds of death increased by 46% (13). However, this tumor is often misdiagnosed as acute or chronic pulmonary embolism because it is characterized by luminal obstruction and intraluminal growth. Gan et al. reported that the wall eclipsing sign on PA-CT angiography is pathognomonic for PAS (14). Endovascular catheter biopsy and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) may be used to diagnose PAS (15). However, EBUS-TBNA increases the risk of bleeding and causes massive hemoptysis. PET-CT has been previously reported to be helpful in diagnostic workup (16). Ito et al. reported that the median maximum standardized uptake value of FDG is 7.95 in PAIS and 2.37 in pulmonary embolism (17). In our case, PET-CT was performed in four cases, with three positive cases. However, Case No. 8 was suspected of having pulmonary embolism because of a negative PET-CT, and was initially treated with heparin, but chest CT after 2 weeks showed no change in the PA intravascular nodule. Therefore, we suspected PAIS and performed the surgery. PAIS should be suspected if there is a large intravascular filling defect and no clinical improvement on anticoagulant therapy. Suto et al. reported that some PAISs with low cellular densities and high mucous tissue proportions have SUVs similar to those in pulmonary thromboembolism (18). Therefore, when we re-evaluated the pathology of Case No. 8, pathologic examination of PAIS revealed low-grade components with myxoid change admixed with high-grade components.
Our study had a few limitations. First, the sample size was small owing to the rarity of the original disease and was a retrospective observational analysis spanning nearly 15 years. In recent years, there have been conspicuous reports that pazopanib is effective for PAIS, and it is possible that the prognosis of patients with PAIS has improved. Second, this study is a single center case series. Therefore, further retrospective research with a higher number of patients at other institutions and a longer follow-up period is necessary.
Conclusions
We may need to perform extended surgery as much as possible and have a distance of at least 20 mm between the location of the tumor on the CT and the surgical incision line during surgery for PAIS. In addition, intensive care such as performing frequent chest CT is required to detect recurrence, considering that there are numerous intrathoracic recurrences with 1 year after surgery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1945/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1945/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1945/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1945/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the medical ethics committee of Juntendo University Hospital (No. 2020115), and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent for this retrospective research was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- A Mandelstamm M. U¨ ber prima¨re neubildungen des herzens. Virchows Arch 1923;245:43-54. [Crossref]
- Grazioli V, Vistarini N, Morsolini M, et al. Surgical treatment of primary pulmonary artery sarcoma. J Thorac Cardiovasc Surg 2014;148:113-8. [Crossref] [PubMed]
- Burke AP, Virmani R. Sarcoma of the great vessels. A clinicopathologic study. Cancer 1993;71:1761-73. [Crossref] [PubMed]
- Fernandez R, Edgren D, Bharat A. Pulmonary Artery Sarcoma. Am J Respir Crit Care Med 2017;195:e23-4. [Crossref] [PubMed]
- Pagni S, Passik CS, Riordan C, et al. Sarcoma of the main pulmonary artery: an unusual etiology for recurrent pulmonary emboli. J Cardiovasc Surg (Torino) 1999;40:457-61. [PubMed]
- Farooki ZQ, Chang CH, Jackson WL, et al. Primary pulmonary artery sarcoma in two children. Pediatr Cardiol 1988;9:243-51. [Crossref] [PubMed]
- Krüger I, Borowski A, Horst M, et al. Symptoms, diagnosis, and therapy of primary sarcomas of the pulmonary artery. Thorac Cardiovasc Surg 1990;38:91-5. [Crossref] [PubMed]
- Blackmon SH, Rice DC, Correa AM, et al. Management of primary pulmonary artery sarcomas. Ann Thorac Surg 2009;87:977-84. [Crossref] [PubMed]
- WHO Classification of Tumours Editorial Board. Thoracic Tumours (5th ed.), WHO classification of tumours series, 5, International Agency for Research on Cancer, Lyon, France, 2021.
- Siordia JA, Garlish A, Truong H. Status postpneumonectomy for pulmonary artery sarcoma. BMJ Case Rep 2015;2015:bcr2015210622. [Crossref] [PubMed]
- Mattoo A, Fedullo PF, Kapelanski D, et al. Pulmonary artery sarcoma: a case report of surgical cure and 5-year follow-up. Chest 2002;122:745-7. [Crossref] [PubMed]
- Kim HK, Choi YS, Kim K, et al. Surgical treatment for pulmonary artery sarcoma. Eur J Cardiothorac Surg 2008;33:712-6. [Crossref] [PubMed]
- Bandyopadhyay D, Panchabhai TS, Bajaj NS, et al. Primary pulmonary artery sarcoma: a close associate of pulmonary embolism-20-year observational analysis. J Thorac Dis 2016;8:2592-601. [Crossref] [PubMed]
- Gan HL, Zhang JQ, Huang XY, et al. The wall eclipsing sign on pulmonary artery computed tomography angiography is pathognomonic for pulmonary artery sarcoma. PLoS One 2013;8:e83200. [Crossref] [PubMed]
- Fujii Y, Koizumi J, Hara T, et al. Endovascular Catheter Biopsy for the Diagnosis of Pulmonary Artery Sarcoma. Ann Vasc Dis 2019;12:256-9. [Crossref] [PubMed]
- Ote EL, Oriuchi N, Miyashita G, et al. Pulmonary artery intimal sarcoma: the role of 18F-fluorodeoxyglucose positron emission tomography in monitoring response to treatment. Jpn J Radiol 2011;29:279-82. [Crossref] [PubMed]
- Ito K, Kubota K, Morooka M, et al. Diagnostic usefulness of 18F-FDG PET/CT in the differentiation of pulmonary artery sarcoma and pulmonary embolism. Ann Nucl Med 2009;23:671-6. [Crossref] [PubMed]
- Suto H, Suto M, Inui Y, Okamura A. Difficulty in Distinguishing Pulmonary Arterial Intimal Sarcoma from Pulmonary Thromboembolism Using FDG PET/CT. In Vivo 2022;36:1519-22. [Crossref] [PubMed]


