
Cytoreductive partial and radical nephrectomies provide equivalent oncologic outcomes in T1–2M1 renal cell carcinoma
Introduction
Renal cell carcinoma (RCC) accounts for approximately 2–3% of all adult malignancies (1). Currently, partial nephrectomy (PN) and radical nephrectomy (RN) are the major surgical methods used in the treatment of RCC, of which PN is mainly performed in T1 cases and selective T2 cases (e.g., in cases with solitary kidney disease or chronic kidney disease or in cases where PN is technically feasible) (2). Some studies have found that PN may also be appropriate for select T3a patients (3,4). PN has been shown to protect renal function from renal failure and cardiovascular events while providing oncologic outcomes similar to radical therapy (5).
While there is general recognition of the role of PN in organ-confined disease, the role of PN in metastatic renal cell carcinoma remains unclear, and RN is still the main means of cytoreductive nephrectomy (CN) (6). This is potentially confusing in patients with a favorable tumor condition locally but with distant metastases.
Most of the previous studies on cytoreductive partial nephrectomy (CPN) suggest that PN provides noninferior or even superior oncologic control compared to RN in these patients. However, the studies are either too old (7,8) or too small in sample size (9), and new evidence is needed in the era of targeted immunotherapy. We took advantage of the large volume of the Surveillance, Epidemiology, and End Results (SEER) database, performed propensity score matching (PSM) to control potential bias, and compared CPN with CRN cytoreductive radical nephrectomy (CRN) in T1–2M1 patients with the aim to provide potentially new guidelines for CN. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1389/rc).
Methods
Launched by the National Cancer Institute, the SEER Program is a population-based cancer database that collects data on incidence, treatment, and mortality of cancer patients across the United States (10). We obtained clinical and pathologic data of patients with RCC from 18 registries between 2010 and 2018 from the SEER database. The patient selection procedure is shown in Figure 1.
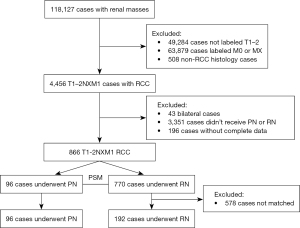
Inclusion criteria for the patients recruited to the study were the following: pathological diagnosis of malignancy; histological confirmation of RCC, including clear cell RCC (ccRCC), RCC not otherwise specified (NOS), papillary RCC (PRCC), acquired cystic disease–associated RCC/tubulocystic RCC, chromophobe RCC (ChRCC), clear cell papillary RCC, collecting duct carcinoma, hereditary leiomyomatosis and RCC (HLRCC), microphthalmia transcription factor (MiT) family translocation RCC (tRCC), mucinous tubular and spindle cell carcinoma, and renal medullary carcinoma); a TNM stage of T1–2M1; no bilateral or other cancers; PN or RN with follow-up >1 month; clear survival status; and known metastatic sites. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
The histologic types were divided into ccRCC and non-ccRCC groups. The demographic and clinicopathological data of the CRN and CPN groups in the study cohort were analyzed. Continuous variables that did not meet the Gaussian distribution were analyzed using the Kruskal-Wallis test, and categorical variables were analyzed using the Fisher exact test. A 1:2 propensity score matching was then performed between the CPN and CRN groups (all clinicopathological variables were used as calipers).
The survival of the 2 groups before and after matching was analyzed with Kaplan-Meier analysis, and the 1-, 3-, and 5-year OS and cancer-specific survival (CSS) of the RN and PN groups were compared with the life table method. Univariate and multivariate Cox regression was then used to analyze the effect of each factor on survival in the matched cohort. The independent variables of multivariate Cox regression were derived from the significant prognostic factors of univariate Cox regression. All the above analyses were performed with R v. 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). A value of P<0.05 was considered statistically significant.
Results
After screening, 866 RCC cases with T1–2M1 were identified (Table 1). The median age of the study cohort was 62 years (22–88 years), and 96 patients (11.1%) received PN as CN. The maximum tumor diameters of the patients with PN were significantly smaller than that of the patients with RN (4.95 vs. 7.5 cm; P<0.001). Although not statistically significant, the 2 groups of cases showed a trend of differences in some other characteristics; specifically, the RN group had a higher proportion of female cases (33.2% vs. 24.0%; P=0.082), a higher proportion of ccRCC (73.5% vs. 63.5%; P=0.052), and a higher proportion of metastases ≥2 (22.3% vs. 14.6%; P=0.087). The median follow-up time was 24 months (1–107 months), and 500 patients (57.7%) were confirmed to have died at the last follow-up, of whom 453 (90.6%) died of RCC. The 1-, 3-, and 5-year survival rates were 76.4%, 48.3%, and 35.1% respectively.
Table 1
| Characteristics | Before match | After match | |||||
|---|---|---|---|---|---|---|---|
| PN (n=96) | RN (n=770) | P | PN (n=96) | RN (n=192) | P | ||
| Age (years), median (IQR) | 64.00 (56.00, 70.00) | 61.00 (54.00, 70.00) | 0.291 | 64.00 (56.00, 70.00) | 62.00 (55.00, 70.25) | 0.946 | |
| Tumor size (cm), median (IQR) | 4.95 (3.50, 7.00) | 7.50 (5.50, 10.00) | <0.001 | 4.95 (3.50, 7.00) | 5.05 (3.80, 6.75) | 0.534 | |
| Race, n (%) | 0.929 | 0.726 | |||||
| Black | 7 (7.3) | 69 (9.0) | 7 (7.3) | 13 (6.8) | |||
| Other | 7 (7.3) | 61 (7.9) | 7 (7.3) | 10 (5.2) | |||
| White | 82 (85.4) | 640 (83.1) | 82 (85.4) | 169 (88.0) | |||
| Sex, n (%) | 0.082 | 0.331 | |||||
| Female | 23 (24.0) | 256 (33.2) | 23 (24.0) | 57 (29.7) | |||
| Male | 73 (76.0) | 514 (66.8) | 73 (76.0) | 135 (70.3) | |||
| Histology, n (%) | 0.052 | 1.000 | |||||
| ccRCC | 61 (63.5) | 566 (73.5) | 61 (63.5) | 123 (64.1) | |||
| Non-ccRCC | 35 (36.5) | 204 (26.5) | 35 (36.5) | 69 (35.9) | |||
| Laterality, n (%) | 0.195 | 1.000 | |||||
| Left | 55 (57.3) | 386 (50.1) | 55 (57.3) | 109 (56.8) | |||
| Right | 41 (42.7) | 384 (49.9) | 41 (42.7) | 83 (43.2) | |||
| T, n (%) | <0.001 | 0.496 | |||||
| T1a | 33 (34.4) | 73 (9.5) | 33 (34.4) | 52 (27.1) | |||
| T1b | 38 (39.6) | 258 (33.5) | 38 (39.6) | 93 (48.4) | |||
| T2a | 18 (18.8) | 239 (31.0) | 18 (18.8) | 34 (17.7) | |||
| T2b | 7 (7.3) | 200 (26.0) | 7 (7.3) | 13 (6.8) | |||
| N, n (%) | 0.147 | 1.000 | |||||
| N0 | 84 (87.5) | 644 (83.6) | 84 (87.5) | 168 (87.5) | |||
| N1 | 7 (7.3) | 102 (13.2) | 7 (7.3) | 13 (6.8) | |||
| NX | 5 (5.2) | 24 (3.1) | 5 (5.2) | 11 (5.7) | |||
| Metastasis, n (%) | 0.087 | 0.734 | |||||
| ≥2 | 14 (14.6) | 172 (22.3) | 14 (14.6) | 32 (16.7) | |||
| 1 | 82 (85.4) | 598 (77.7) | 82 (85.4) | 160 (83.3) | |||
| Grade, n (%) | 0.640 | 0.961 | |||||
| I | 1 (1.0) | 21 (2.7) | 1 (1.0) | 2 (1.0) | |||
| II | 22 (22.9) | 217 (28.2) | 22 (22.9) | 43 (22.4) | |||
| III | 41 (42.7) | 277 (36.0) | 41 (42.7) | 75 (39.1) | |||
| IV | 18 (18.8) | 138 (17.9) | 18 (18.8) | 39 (20.3) | |||
| Unknown | 14 (14.6) | 117 (15.2) | 14 (14.6) | 33 (17.2) | |||
| Radiation, n (%) | 1.000 | 0.897 | |||||
| None/unknown | 61 (63.5) | 490 (63.6) | 61 (63.5) | 119 (62.0) | |||
| Yes | 35 (36.5) | 280 (36.4) | 35 (36.5) | 73 (38.0) | |||
| Systemic therapy, n (%) | 0.332 | 0.612 | |||||
| No/unknown | 54 (56.2) | 392 (50.9) | 54 (56.2) | 115 (59.9) | |||
| Yes | 42 (43.8) | 378 (49.1) | 42 (43.8) | 77 (40.1) | |||
RCC, renal cell carcinoma; ccRCC, clear cell renal cell carcinoma; PN, partial nephrectomy; PSM, propensity score matching; RN, radical nephrectomy; IQR, interquartile range.
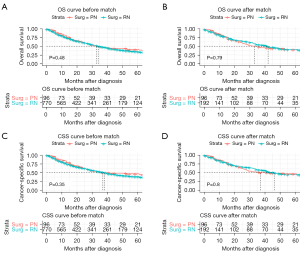
Kaplan-Meier analysis showed that OS (P=0.48) and CSS (P=0.35) in the PN group were not significantly different from those in the RN group in the study cohort (Figure 2). After PSM, all clinicopathologic characteristics of cases in the PN and RN groups were comparable (Table 1), and there was still no significant difference in OS (P=0.79) and CSS (P=0.80) between the 2 groups after matching (Figure 2).
Univariate and multivariate Cox regression analysis showed that the risk factors for OS were higher age [hazard ratio (HR) =1.02; P=0.008], non-ccRCC pathological type (HR =1.69, P=0.002), number of metastases ≥2 (HR =2.13; P<0.001), and regional lymph node involvement (HR =2.22; P=0.004); and the risk factors for CSS were non-ccRCC pathological type (HR =1.51; P=0.021) and number of metastases ≥2 (HR =2.24; P<0.001). The surgical method had no significant impact on OS or CSS in the study cohort, and age was a risk factor for OS but not for CSS. Tumor maximum diameter, nuclear grade, and systemic therapy showed no differences in the impact on OS and CSS. Among the risk factors for OS and CSS, metastatic burden appears to be the most dominant (Tables 2,3).
Table 2
| Characteristics | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| Age (year) | 1.02 | 1–1.03 | 0.047 | 1.02 | 1.01–1.04 | 0.008 | |
| Histology | 0.004 | 0.002 | |||||
| ccRCC | 1 (reference) | 1 (reference) | |||||
| Non-ccRCC | 1.6 | 1.16–2.21 | 1.69 | 1.22–2.34 | |||
| Metastasis | <0.001 | <0.001 | |||||
| 1 | 1 (reference) | 1 (reference) | |||||
| ≥2 | 2.08 | 1.39–3.1 | 2.13 | 1.42–3.20 | |||
| N stage | 0.021 | 0.028 | |||||
| N0 | 1 (reference) | 1 (reference) | |||||
| N1 | 2.25 | 1.31–3.84 | 0.003 | 2.22 | 1.28–3.83 | 0.004 | |
| NX | 1.36 | 0.73–2.52 | 0.330 | 1.33 | 0.71–2.49 | 0.372 | |
OS, overall survival; CI, confidence interval; RCC, renal cell carcinoma; ccRCC, clear cell renal cell carcinoma.
Table 3
| Characteristics | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| Histology | 0.027 | 0.021 | |||||
| ccRCC | 1 (reference) | 1 (reference) | |||||
| Non-ccRCC | 1.48 | 1.05–2.1 | 1.51 | 1.06–2.15 | |||
| Metastasis | <0.001 | <0.001 | |||||
| 1 | 1 (reference) | 1 (reference) | |||||
| ≥2 | 2.25 | 1.48–3.42 | 2.24 | 1.46–3.14 | |||
| N stage | 0.021 | 0.086 | |||||
| N0 | 1 (reference) | 1 (reference) | |||||
| N1 | 2.23 | 1.25–3.98 | 0.006 | 1.99 | 1.11–3.57 | 0.020 | |
| NX | 1.47 | 0.77–2.81 | 0.246 | 1.3 | 0.68–2.49 | 0.435 | |
CSS, cancer-specific survival; CI, confidence interval; RCC, renal cell carcinoma; ccRCC, clear cell renal cell carcinoma.
Discussion
Metastatic disease is the leading cause of death in RCC, and the median OS of metastatic RCC is only 45.7 months even under the most leading-edge systemic therapy (11). Nephrectomy can be expected to achieve curative effect in both localized and locally advanced RCC, but it is not satisfactory in metastatic RCC. The CARMENA study showed that the OS of sunitinib alone in patients with mRCC was not inferior to cytoreductive nephrectomy plus sunitinib [HR =0.97; 95% confidence interval (CI): 0.79–1.19; P=0.8] and could even yield longer OS (19.8 vs. 15.6 months); the study also demonstrated that CN can play a role in patients with less metastatic burden (11). The SURTIME study reported that delayed CN did not improve progression-free survival compared with immediate CN followed by sunitinib (43% vs. 42%; P=0.61), but delayed CN achieved better OS in the intention-to-treat (ITT) population (HR =0.57, 95% CI: 0.34–0.95; P=0.03) (6). Delayed CN can also be used in people who benefit from systemic therapy and in those in good physical condition who do not need systemic therapy (2).
Although CN remains controversial, it remains an important option for the treatment of metastatic RCC in clinical practice (2). Out of habitual thinking, urologists mostly choose RN for patients with metastatic RCC even though some patients have a more favorable local condition (12-14). This led us to wonder whether CPN could preserve renal function while achieving noninferior oncologic outcomes compared to CRN in these patients. In fact, some researchers have already considered this possibility. Capitanio et al. analyzed data in the SEER database from 1988 to 2004 and found that CSS in the CPN group was not worse than that in the CRN group (HR =1.40; P=0.16), but they did not limit the local condition (T stage) (15). Working from this basis, Lenis et al., who analyzed data in the SEER database from 2006 to 2013, found an upward trend in the proportion of patients with mRCC receiving CPN. Additionally, patients presenting in academic/research institutions were more likely to receive CPN [odds ratio (OR) =1.44; 95% CI: 1.12–1.85; P=0.004], and in cases with tumors smaller than 4 cm, CPN was associated with better OS (HR =0.81; 95% CI: 0.71–0.93; P=0.002) (16).
We analyzed more recent data than reported in the above studies and found that both systemic therapy and the PN technique have improved over the past few years (17); thus, our included cases received treatment regimens that were closer to current guidelines and, therefore, more stringent screening criteria were applied, and we included the variable number of metastases, which proved to be the most significant prognostic factor for mRCC in our study.
In addition to reliable long-term oncologic outcomes, patients who received CPN had acceptable perioperative outcomes. Babaian et al. (9) reviewed the perioperative data and survival outcomes of 33 patients with mRCC who received CPN in a single center (patients with adrenal invasion were also included, as this is classified as local involvement rather than distant metastasis according to the current TNM staging system). A total of 17 postoperative complications occurred in 12 patients, 6 of whom were graded higher than Clavien III. All patients recovered after treatment. In this cohort, 22 patients died of RCC after a median follow-up of 27 months postsurgery. Unfortunately, there were no CRN cases to serve as controls.
Theoretically, there are some disadvantages of PN compared to RN. One is the possible occurrence of positive surgical margins leading to local recurrence; however, whether positive surgical margins lead to worse tumor outcomes remains controversial. A study by Petros et al. showed that positive surgical margins in patients with PN were associated with recurrence, metastasis, and worse survival (18), but Tabayoyong et al., Takagi et al., and Kang et al. found that positive surgical margins in patients with PN did not definitively translate into worse oncologic outcomes (19-21). The current European Association of Urology (EAU) guidelines only recommend intensive follow-up for patients with positive margins (2). Moreover, the significance of a positive surgical margin in CN has not been found in those with mRCC. The advantages of CPN are obvious. Although it fails to significantly improve the oncologic outcomes of patients, it is significantly better than CRN in terms of preserving renal function and improving quality of life (22,23).
Some studies have suggested that CPN results in better survival than does CRN (16,24), yet we are skeptical about this. A study by Palacios et al. found that adverse tumor outcomes were related to the aggressive nature of the tumor rather than the degree of preservation of renal function (25). The retrospective study design and some unavoidable selection biases might have skewed the findings in favor of CPN.
In our study, there were also some limitations, such as the inability to obtain information on the patients’ physical condition, body mass index (BMI), and laboratory data (such as hemoglobin, albumin, and lactate dehydrogenase). Beyond this, information on systemic treatment in the SEER database includes details of chemotherapy, targeted therapy, and immunotherapy, but the inability to distinguish between these treatments in the SEER database and the lack of access to a patient’s specific regimen of systemic therapy might also have produced bias. The International mRCC Database Consortium (IMDC) or Memorial Sloan-Kettering Cancer Center (MSKCC) risk scores may also interfere with a physician’s selection of CPN or CRN, but information on these scores was also lacking in the database. In addition, we noticed that the proportion of patients who accepted systemic therapy was only 40%, which may be related to the database’s preference for recording hospitalization and surgical cases. Today, systemic therapy is the preferred first-line treatment for mRCC and treatment is more diverse, which may limit the value of this study.
Conclusions
CPN provides similar survival to CRN in select patients with mRCC, and tumor metastatic burden is the most significant prognostic factor for mRCC. However, due to the retrospective design of this study, further research is still needed to verify these results before CPN can be used in clinical practice.
Acknowledgments
The authors would like to thank Professor Shaogang Wang (Department of Urology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology) for data curation.
Funding: This work was supported by National Natural Science Foundation of China (No. 81702989).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1389/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1389/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [Crossref] [PubMed]
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol 2022;82:399-410. [Crossref] [PubMed]
- Yim K, Aron M, Rha KH, et al. Outcomes of Robot-assisted Partial Nephrectomy for Clinical T3a Renal Masses: A Multicenter Analysis. Eur Urol Focus 2021;7:1107-14. [Crossref] [PubMed]
- Tian J, Zeng X, Wan J, et al. Partial and Radical Nephrectomy Provides Equivalent Oncologic Outcomes in pT3a Renal Cell Carcinoma: A Population-Based Study. Front Oncol 2022;11:819098. [Crossref] [PubMed]
- Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 2010;183:1317-23. [Crossref] [PubMed]
- Bex A, Mulders P, Jewett M, et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol 2019;5:164-70. [Crossref] [PubMed]
- Hellenthal NJ, Mansour AM, Hayn MH, et al. Is there a role for partial nephrectomy in patients with metastatic renal cell carcinoma? Urol Oncol 2013;31:36-41. [Crossref] [PubMed]
- Karam JA, Babaian KN, Tannir NM, et al. Role of partial nephrectomy as cytoreduction in the management of metastatic renal cell carcinoma. Minerva Urol Nefrol 2015;67:149-56. [PubMed]
- Babaian KN, Merrill MM, Matin S, et al. Partial nephrectomy in the setting of metastatic renal cell carcinoma. J Urol 2014;192:36-42. [Crossref] [PubMed]
- Cronin KA, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer 2014;120:3755-7. [Crossref] [PubMed]
- Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 2020;21:1563-73. [Crossref] [PubMed]
- Méjean A, Ravaud A, Thezenas S, et al. Sunitinib Alone or After Nephrectomy for Patients with Metastatic Renal Cell Carcinoma: Is There Still a Role for Cytoreductive Nephrectomy? Eur Urol 2021;80:417-24. [Crossref] [PubMed]
- Bhindi B, Abel EJ, Albiges L, et al. Systematic Review of the Role of Cytoreductive Nephrectomy in the Targeted Therapy Era and Beyond: An Individualized Approach to Metastatic Renal Cell Carcinoma. Eur Urol 2019;75:111-28. [Crossref] [PubMed]
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171:1071-6. [Crossref] [PubMed]
- Capitanio U, Zini L, Perrotte P, et al. Cytoreductive partial nephrectomy does not undermine cancer control in metastatic renal cell carcinoma: a population-based study. Urology 2008;72:1090-5. [Crossref] [PubMed]
- Lenis AT, Salmasi AH, Donin NM, et al. Trends in usage of cytoreductive partial nephrectomy and effect on overall survival in patients with metastatic renal cell carcinoma. Urol Oncol 2018;36:78.e21-8. [Crossref] [PubMed]
- Andrade HS, Zargar H, Caputo PA, et al. Five-year Oncologic Outcomes After Transperitoneal Robotic Partial Nephrectomy for Renal Cell Carcinoma. Eur Urol 2016;69:1149-54. [Crossref] [PubMed]
- Petros FG, Metcalfe MJ, Yu KJ, et al. Oncologic outcomes of patients with positive surgical margin after partial nephrectomy: a 25-year single institution experience. World J Urol 2018;36:1093-101. [Crossref] [PubMed]
- Takagi T, Yoshida K, Wada A, et al. Predictive factors for recurrence after partial nephrectomy for clinical T1 renal cell carcinoma: a retrospective study of 1227 cases from a single institution. Int J Clin Oncol 2020;25:892-8. [Crossref] [PubMed]
- Kang HW, Lee SK, Kim WT, et al. Surgical margin does not influence recurrence rate in pT1 clear cell renal cell carcinoma after partial nephrectomy: A multicenter study. J Surg Oncol 2016;114:70-4. [Crossref] [PubMed]
- Tabayoyong W, Abouassaly R, Kiechle JE, et al. Variation in Surgical Margin Status by Surgical Approach among Patients Undergoing Partial Nephrectomy for Small Renal Masses. J Urol 2015;194:1548-53. [Crossref] [PubMed]
- Lesage K, Joniau S, Fransis K, et al. Comparison between open partial and radical nephrectomy for renal tumours: perioperative outcome and health-related quality of life. Eur Urol 2007;51:614-20. [Crossref] [PubMed]
- MacLennan S, Imamura M, Lapitan MC, et al. Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur Urol 2012;62:1097-117. [Crossref] [PubMed]
- Chen J, He Q, Liu W, et al. The Effect of Cytoreductive Partial Nephrectomy in Elderly Patients with Metastatic Renal Cell Carcinoma. Clin Interv Aging 2020;15:431-9. [Crossref] [PubMed]
- Palacios DA, Zabor EC, Munoz-Lopez C, et al. Does Reduced Renal Function Predispose to Cancer-specific Mortality from Renal Cell Carcinoma? Eur Urol 2021;79:774-80. [Crossref] [PubMed]


