
Intracranial complete remissions in an aumolertinib-treated EGFR mutation-positive non-small cell lung cancer (NSCLC) patient with symptomatic brain metastases and Eastern Cooperative Oncology Group performance status (ECOG PS) up to 4: a case report
Highlight box
Key findings
• There is no extensive research data available for symptomatic brain metastases and high ECOG PS score in EGFR mutation-positive NSCLC patient. However, our case firstly presented an aumolertinib-treated patient with symptomatic brain metastases obtained intracranial complete remissions and ECOG PS score down from 4 to 0.
What is known and what is new?
• Patients with brain metastases have low quality of life and poor overall survival;
• Aumolertinib, the third generation EGFR-TKI originally in China, has high brain exposure and strong inhibition of brain metastases. In this case, multiple brain metastases completely disappeared and PS score returned to 0 with no apparent adverse effects (AEs)
What is the implication, and what should change now?
• This case shows confidence in aumolertinib monotherapy as a promising treatment option for EGFR-positive NSCLC with brain metastasis. Further comprehensive studies with a large population are required to understand its efficacy and AEs.
Introduction
The incidence of brain metastasis in epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC) is relatively high compared to EGFR wild type (70% vs. 38%) (1). A study based on Surveillance, Epidemiology, and End Results (SEER) data showed a low median survival of 2.9 months among patients diagnosed with brain metastases (2). EGFR-tyrosine kinase inhibitors (TKIs) could be the best treatment option for EGFR-mutant cancer patients, especially for central nervous system (CNS) metastasis (3). National Comprehensive Cancer Network (NCCN) recommends third-generation EGFR-TKI as first-line therapy for EGFR mutation-positive patients (4). Aumolertinib is a novel, third-generation EGFR-TKI that demonstrated high activity against EGFR sensitizing mutations, EGFR T790M mutation, and CNS metastases (5). Here we report an EGFR mutation-positive NSCLC patient with symptomatic brain metastases and an Eastern Cooperative Oncology Group performance status (ECOG PS) up to 4 successfully treated with aumolertinib. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1614/rc).
Case presentation
A 74-year-old male smoker presented in our clinic with a history of one-day dizziness, two hours of convulsions in the right lower limb, nausea, and excessive vomiting. He has a normal blood pressure (118/78 mmHg) and an elevated plasma carcinoembryonic antigen (CEA) level of 61.75 ng/mL. Chest computed tomography (CT) suggested hilar nodules in the left lung, mediastinal lymph node enlargement, and multiple small nodules in both lungs. Emergency brain CT scans identified multiple brain metastasis, and numerous metastases were diagnosed with the help of magnetic resonance imaging (MRI). Cervical lymph node biopsy revealed adenocarcinoma. Overall, he has been diagnosed with stage IV malignancy, and the EGFR gene sequencing analysis showed the deletion of exon 19.
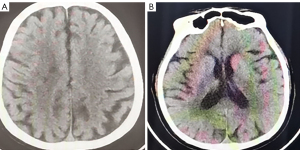
Symptomatic supportive and anti-tumor therapy were given to the patient. The regimen consists of one cycle of aumolertinib 110 mg and one cycle of bevacizumab, followed by aumolertinib monotherapy. Currently, the patient is still taking a maintenance dose of aumolertinib 110 mg daily and is regularly being followed up. On August 7, 2020, the patient had no seizures, noticeable nausea and vomiting, or occasional dizziness; his right lower limb muscle strength was still graded 4, but he attained muscular strength. A week later, his PS score returned to 0 with no apparent adverse effects (AEs). Two months later, the lesion in the left lung lesion was significantly reduced, and the mediastinal lymph nodes were shrunk considerably (Figure 1); efficacy assessment showed that cancer in the patient reached partial remissions (PR). Meanwhile, multiple brain metastases were significantly reduced and partially disappeared (Figure 2), and cerebral edema completely disappeared (Figure 3). Three months later, his CEA levels were decreased to 5.61 ng/mL, and multiple brain metastases completely disappeared (Figure 4); intracranial curative effect reached complete remissions (CR).
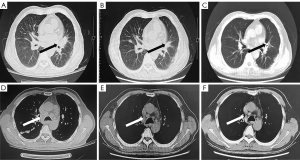
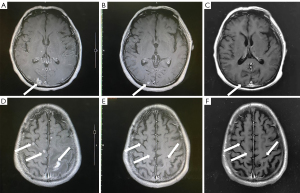
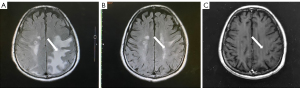
On September 17th 2021, 14 months after initial admission, the lung CT revealed a shrunken lesion (Figure 1). A brain MRI showed a complete absence of multiple brain metastases and cerebral edema (Figures 2,3). The patient is still alive, 26 months since diagnosis without apparent AEs and living a normal life. He was still under treatment and followed up at the time of writing.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Our patient had an ECOG PS score of 4, and genetic analysis detected a deletion of exon 19 in the EGFR gene. The EGFR mutation-positive advanced NSCLC with baseline PS 2 to 4 have a mortality rate 61% higher than other NSCLCs (6). Several medicines have been studied in EGFR mutation-positive or other NSCLC via randomized controlled trials (RCTs). The patients with specific characteristics commonly observed in clinical practice are usually excluded in RCTs, such as brain metastases, primarily symptomatic, or ECOG PS no less than 2 (7). Study on using EGFR-TKIs in symptomatic brain metastases or combining with stereotactic radiosurgery is also limited as patients included in the trials had already been treated by EGFR-TKIs or asymptomatic brain metastases (8). Therefore, there is currently no effective treatment regimen for EGFR-positive NSCLC patients with symptomatic brain metastases and ECOG PS scores up to 4.
In the APOLLO study, aumolertinib demonstrated outstanding clinical efficacy against CNS metastases, with CNS objective response rate (ORR) and CNS disease control rate (DCR) of 60.9% and 91.3%, respectively, as well as a CNS median progression-free survival (PFS) and median duration of response (DOR) of 10.8 and 12.5 months respectively (5).
Thus, we administered combined therapy for brain metastases, including bevacizumab and aumolertinib. The patient, without symptoms of hypertension, was discharged from the hospital 2 weeks after aumolertinib. He then received aumolertinib 110 mg daily at home. Since bevacizumab needed to be injected in the hospital, and the disease had been well controlled by aumolertinib monotherapy, and PR was obtained in 6 weeks, whereas bevacizumab was given only once. To the best of our knowledge, we are reporting for the first time that a patient with brain metastasis and PS score four obtained PR/CR through aumolertinib plus bevacizumab, followed by aumolertinib monotherapy without progression or obvious AEs. Thus, this case provides confidence in using aumolertinib monotherapy as a promising treatment option for EGFR-positive NSCLC with metastasis. Further comprehensive studies with a large population are required to understand its efficacy, efficiency, and AEs.
Acknowledgments
The authors thank the patient for participating and agreeing to publish the report.
Funding: The project was supported by Wu Jieping Medical Fund (No. 2021HZ020).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1614/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1614/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1614/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ge M, Zhuang Y, Zhou X, et al. High probability and frequency of EGFR mutations in non-small cell lung cancer with brain metastases. J Neurooncol 2017;135:413-8. [Crossref] [PubMed]
- Lamba N, Kearney RB, Catalano PJ, et al. Population-based estimates of survival among elderly patients with brain metastases. Neuro Oncol 2021;23:661-76. [Crossref] [PubMed]
- Weber B, Winterdahl M, Memon A, et al. Erlotinib accumulation in brain metastases from non-small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J Thorac Oncol 2011;6:1287-9. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Non-Small Cell Lung Cancer Clinical Practice Guidelines Version 3. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed March 16, 2022).
- Lu S, Wang Q, Zhang G, et al. Efficacy of Aumolertinib (HS-10296) in Patients With Advanced EGFR T790M+ NSCLC: Updated Post-National Medical Products Administration Approval Results From the APOLLO Registrational Trial. J Thorac Oncol 2022;17:411-22. [Crossref] [PubMed]
- Kato Y, Hosomi Y, Watanabe K, et al. Impact of clinical features on the efficacy of osimertinib therapy in patients with T790M-positive non-small cell lung cancer and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J Thorac Dis 2019;11:2350-60. [Crossref] [PubMed]
- de Marinis F, Laktionov KK, Poltoratskiy A, et al. Afatinib in EGFR TKI-naïve patients with locally advanced or metastatic EGFR mutation-positive non-small cell lung cancer: Interim analysis of a Phase 3b study. Lung Cancer 2021;152:127-34. [Crossref] [PubMed]
- Bhandari S, Dunlap N, Kloecker G. Radiotherapy in brain metastases from EGFR-mutated non-small cell lung cancer. J Thorac Dis 2021;13:3230-4. [Crossref] [PubMed]


