
A diagnostic challenge of mysterious bone pain caused by tumor-induced osteomalacia with multiple tumors: a case report
Highlight box
Key findings
• As a rare disease, tumor-induced osteomalacia (TIO) is highly susceptible to misdiagnosis. Electrolyte testing and appropriate imaging techniques are crucial for TIO diagnosis.
What is known and what is new?
• In most cases, TIO can be effectively treated; however, it is difficult to diagnose.
• This case report emphasizes the importance of electrolyte testing for quickly identifying TIO and the use of appropriate imaging techniques (such as 68Ga-DOTATATE PET/CT) for locating TIO lesions.
What are the implications of this study, and what should change now?
• Clinicians should suspect TIO when a patient is experiencing bone pain with an unknown primary cause. Electrolyte testing is useful for primary identification of TIO and appropriate imaging techniques are necessary for tumor localization. For researchers, large-scale epidemiological investigations are necessary to explore the clinical characteristics of TIO in order to accelerate the diagnosis of TIO in clinical practice.
Introduction
Tumor-induced osteomalacia (TIO) is a rare, paraneoplastic syndrome in which the patient presents with acquired hypophosphatemic osteomalacia. The clinical characteristics of TIO are very similar to those of genetic hypophosphatemic rickets (1-3). Previous research has indicated that tumor-induced humoral factors play a crucial role in the pathogenesis of TIO (4). For patients who are eligible for surgery, TIO can be completely cured by resecting the tumor (2), while pharmacological interventions are useful for patients for whom surgery is not possible (5).
However, despite the treatability of TIO, its diagnosis and differential diagnosis remain a clinical challenge, in part due to its frequency and insidiousness (6). In addition, patients can present with multiple diseases simultaneously, which may hinder the discovery of the real etiology by clinicians. Therefore, relevant clinical reports will deepen the understanding of the clinical characteristics of TIO and accelerate its clinical diagnosis.
Herein, we describe a case of TIO in which the diagnostic workup was complicated by the rarity of TIO and the presence of other abnormal disorders in the patient. The information in this case is potentially useful for other clinicians considering a differential diagnosis of TIO. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2116/rc).
Case presentation
A 52-year-old Chinese woman presented to the Department of Integrated Traditional Chinese and Western Medicine of Tongji Hospital with a 1-year history of generalized weakness and multiple bone and joint pain. The patient had no significant past medical history and no medical history of rheumatism or neurological disorders. No identifiable patient or imaging data are used in this report. All procedures in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent form is available for review by the editorial office of this journal.
The timeline of the case is illustrated in Figure 1. More than year ago, the patient began to experience pain in her anterior chest, back, waist, bilateral hip joints, and ankle joints with no significant triggers. The pain especially intensified when turning over in bed but was usually within a tolerable range. The patient had no symptoms of swelling, deformation, or stiffness of joints in the morning, and no fever, skin rashes, mouth ulcers, hair loss, dry mouth and eyes, or symptoms of Raynaud’s phenomenon. The patient’s symptoms grew progressively worse in the year leading up to her presentation at our hospital. The patient reported experiencing poor sleep and lower levels of physical activity after the onset of her symptoms, but her appetite, urination, and stools were normal.
The patient visited the local hospital. Computed tomography (CT) scans of the craniocerebral and sacroiliac joints suggested there were no significant abnormalities. The patient underwent rehabilitation at the local hospital, but there were no beneficial therapeutic effects. Subsequently, after experiencing worsening of her symptoms, the patient was referred to our hospital.
Physical examination at admission revealed a blood pressure of 116/79 mmHg, a pulse rate of 93 beats per minute, a respiratory rate of 20 breaths per minute, and a body temperature of 36.5 ℃. No abnormal signs were found on the chest or abdominal physical examinations. During the neurological and joint examinations, the patient exhibited limited joint mobility when walking or turning around. The patient had no spinal ankylosis and no joint swelling or deformation. The straight leg raise test and Patrick’s sign were negative. Physical examination showed that the muscle strength of the upper and lower extremities was grade V and grade IV, respectively.
After an integrated consideration of the patient’s medical history and physical examination results, we initially suspected that a rheumatic disease or the osteoarticular system was responsible for her pain. Laboratory examinations were conducted; however, no obvious abnormalities were detected via routine blood tests, urine tests, or blood biochemistry analysis, except for elevated uric acid and alkaline phosphatase levels. Four blood electrolytes (K, Na, Cl, and Ca) were also tested, and all were normal. In addition, rheumatological laboratory test results, including those for rheumatoid factor, anti-cyclic citrullinated peptide antibodies, and anti-nuclear antibodies, were all negative. Inflammatory and infectious indicators, including the erythrocyte sedimentation rate, C-reactive protein, and procalcitonin, also showed no abnormalities. These results indicated that the patient’s symptoms might not be caused by rheumatic diseases. Furthermore, a negative test result for human leukocyte antigen B27 reduced the likelihood of the patient having ankylosing spondylitis.
Considering that the patient may have a disorder of the osteoarticular system, we applied several imaging examinations. A pelvic radiograph revealed degeneration of the pelvis and the bilateral hip (Figure 2). Osteosclerosis could be seen in the iliac of the sacroiliac joints, and the local joint space was unclear. Magnetic resonance imaging (MRI) of the lumbar spine indicated an abnormal signal in the third lumbar vertebra (L3) potentially caused by hemangioma. A whole-body bone scan by emission CT indicated that multiple joints had lesions, which were potentially caused by metabolic bone disease or inflammatory changes. However, chest CT also suggested that there was a soft tissue nodule in the anterior right mediastinum (18 mm × 10 mm). Therefore, an MRI of the mediastinum was performed, and the result showed that the nodule was a tumor potentially derived from the thymus. Given that the patient had severe muscle weakness and potential thymic carcinoma, we initially suspected that her symptoms were caused by thymomatous myasthenia gravis; however, the patient lacked typical symptoms of myasthenia gravis, such as blepharoptosis and dysphagia. A subsequent electromyography investigation indicated that the patient’s peripheral neuromuscular junction was normal, which meant that myasthenia gravis was unlikely to be the reason for the weakness.

As the patient was in the perimenopausal period and presented with an abnormal whole-body bone scan by emission CT, osteoporosis was considered as another possible cause of her symptoms. Serum 25-hydroxyvitamin D, osteocalcin, total procollagen-type I amino-terminal propeptide, carboxy-terminal cross-linked telopeptide of type I collagen, serum calcium, and serum phosphorous were detected simultaneously. Laboratory tests showed that the patient had lower 25-hydroxyvitamin D (11.6 ng/mL) and serum phosphorous (0.35 mmol/L) than the reference intervals. Furthermore, bone mineral density accessed by dual energy X-ray absorptiometry also showed osteopenia. In combination, these results indicated that the patient’s symptoms were probably caused by osteoporosis; however, considering the patient was in the perimenopausal period, her symptoms were less likely to be caused by primary osteoporosis due to sudden systemic illness.
We firstly excluded multiple myeloma based on negative monoclonal immunoglobulin protein test findings, and we later found that the patient had low phosphorus-associated osteoporosis. This finding suggested that the patient might have hypophosphatemic osteomalacia, a rare and not easily diagnosable metabolic disease. This syndrome can be caused by genetic disorders, including X-linked hypophosphatemia and autosomal dominant hypophosphatemic rickets, or induced by specific tumors or drugs (7). Considering the patient’s lack of relevant family history, her hypophosphatemic osteomalacia was likely to be an acquired disorder. Normokalemia further excluded Fanconi syndrome. Subsequently, a potential diagnosis of TIO was considered. From the MRI findings, we suspected that there was a tumor in the mediastinum; however, its location could not be definitively identified.
Somatostatin receptor 2A is expressed in phosphaturic mesenchymal tumors (PMTs) and has been reported to be closely associated with TIO (8). Gallium-68 dotatate (68Ga-DOTATATE) is a somatostatin analog imaging agent widely used to identify the location of TIO lesions in positron emission tomography (PET/CT). A meta-analysis suggested that imaging modalities based on somatostatin receptors outperformed F-fluorodeoxyglucose (F-FDG) PET/CT in the detection of TIO, with 68Ga-DOTA-conjugated-somatostatin-receptor-targeting-peptides (68Ga-DOTA-SST) PET/CT performing slightly better than Octreoscan single-photon emission CT (SPECT)/CT (9). Therefore, PET/CT and 68Ga-DOTATATE PET/CT were performed to find all potential tumors in our patient. The PET/CT results indicated that there were two neoplasms, one in the left nasal cavity and one in the mediastinum, and DOTATATE PET/CT showed that the neoplasm in the left nasal cavity had a higher uptake of DOTATATE. This finding suggested that the ultimate cause of the TIO was the tumor in the left nasal cavity (Figure 3).
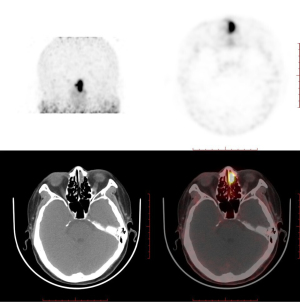
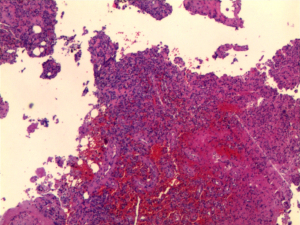
The patient was administered bisphosphonate plus a combination of calcium and vitamin D orally after being diagnosed with osteoporosis, which slightly ameliorated her symptoms. Once TIO was determined to be the cause of the osteoporosis, the patient agreed to undergo surgery to excise the lesion. Spindle cell proliferation and pathological characteristics of anaplastic hemangiopericytomas were observed on hematoxylin and eosin staining of the neoplasm, while the exact calcium salt deposition in the hematoxylin and eosin-stained section was not determined. Immunohistochemical staining showed that CD56, CD31, CD34, ETS (erythroblast transformation-specific)-related gene, and Syn were positive, and the percentage of KI67-positive cells was 1–2%. Pathological changes and clinical manifestations suggested that the neoplasm was potentially a PMT (Figure 4). After surgery, the patient experienced a good recovery, with relief of her bone pain and other symptoms. On postoperative day 3, the patient’s blood phosphorus increased to 0.78 mmol/L. Although her electrolyte levels were elevated and had not returned to normal, the patient hoped to leave hospital as soon as possible and was discharged. The patient was followed up via outpatient clinics at 1 and 2 months after surgery, and her blood phosphorus levels returned to within the normal reference interval (0.97 mmol/L at 1 month and 1.06 mmol/L at 2 months after surgery). The patient self-reported that almost all her symptoms had disappeared, and she regained the ability to live independently. The patient was grateful for ultimately receiving a definitive diagnosis and effective treatment despite enduring ongoing illness for more than 1 year.
Discussion
TIO, also known as oncogenic osteomalacia, is a rare paraneoplastic syndrome. Currently, there are few relevant reports on the global incidence of TIO. A recent study in Denmark reported the incidence of TIO among the total population of the country to be less than 0.13 per 100,000 person-years (10). Mechanistically, tumor-derived fibroblast growth factor 23 (FGF23) plays an important role in the pathological processes of TIO by inducing the release of phosphate and calcitriol deficiency, which ultimately leads to severe bone loss (11-13). The clinical manifestations of TIO are mainly characterized by weakness and pain in the extremities in adults and by rickets in children (14,15). According to clinical reports, TIO can be cured through surgery (2,3). For patients with TIO who are not eligible candidates for surgery, treatment with drugs, including burosumab, may control the condition (5). Although TIO does not have high incidence or mortality, its delayed diagnosis is a key issue that needs to be urgently resolved, as the syndrome severely limits the mobility of patients and impairs their quality of life (16,17). However, the low incidence and prevalence of TIO limits large-scale clinical reporting of the syndrome. As an alternative, single-case studies can promote the understanding and accumulation of the diagnostic experience of TIO, albeit inefficiently.
Connections between TIO and PMT were first established in the 1950s. Approximately 80% of cases of TIO are caused by PMT, while the other 20% of cases are attributable to other types of mesenchymal tumor, including hemangiopericytoma, giant cell tumor of bone, and osteosarcoma. Cases of PMT presenting in the head and neck region are relatively rare. A case review showed that there were only 71 cases of head and neck PMT published between 1972 and 2015 (18). According to the case review, among patients with head and neck PMT, there are more females than males. Improving the understanding of the focal sites of tumors would support the diagnosis of PMT and TIO, as PMT in the head and neck tends to appear at extraoral sites instead of intraoral sites. The paranasal sinuses and mandibles are the two most common sites of head and neck PMT, according to the literature review. Most cases of head and neck PMT are benign; however, in a small number of cases, head and neck PMT recurs, and in a very small number of cases, metastases are observed, which affects the prognosis of patients and warrants the attention of clinicians. The typical histological features of PMT can be summarized as bland spindle cells with vascularity and numerous multinucleated giant cells embedded in a chondromyxoid matrix with focal areas of calcification. However, due to their lack of specificity, histological features alone are not suitable for making a diagnosis. However, serum or tumor testing to detect FGF23 protein or mRNA expression, as well as PET/CT, are beneficial for the diagnosis of PMT and TIO.
Overall, elucidation of the clinical characteristics, anatomical location, and histological characteristics of head and neck PMT and its induced TIO are pending further investigation due to their very low incidence rates. Reviewing past published cases is a commonly used approach to understanding the clinical features of a disease, especially when the incidence is low. However, the results of the literature review may not be accurate due to multiple potential biases. Therefore, to fully and accurately investigate PMT, retrospective epidemiological studies that target an entire district and adequate explorative clinical studies are called for in the future.
In this paper, we described a patient with TIO who presented with bone pain and muscle weakness and a suspected rheumatic disease or thymic-derived tumorous lesion accompanied by myasthenia gravis. Subsequently, we found that the patient had osteoporosis accompanied by hypophosphatemia. Ultimately, the patient was diagnosed with TIO via 68Ga-DOTATATE PET/CT imaging. After undergoing surgery, the patient recovered and showed signs of improvement at the postoperative 1- and 2-month follow-ups. Based on this case, we believe that clinicians should take into account the clinical characteristics of TIO, despite its rarity, and that multidisciplinary collaboration is necessary for the differential diagnosis of TIO. Moreover, we can infer two recommendations from our experience with this case. First, patients who present with clinical characteristics similar to those in our patient should also be evaluated for hypophosphatemia by screening blood electrolyte levels to assist clinicians in their initial screening. Secondly, locating and identifying the lesion responsible for the TIO is extremely important. The same patient may have several tumors; therefore, clinicians should be alert as to whether a patient with TIO has several tumors. Locating all tumors by CT or MRI is not easy, but PET/CT is able to identify all potential tumors, and 68Ga-DOTATATE PET/CT is a powerful diagnostic tool for locating the true culprit of TIO (19,20).
In summary, few reports have focused on patients with TIO whose diagnosis is impaired by the presence of multiple tumors. Our patient was suspected to have thymoma caused by a mediastinal lesion. The patient was ultimately diagnosed with TIO which contributed to osteoporosis and hypophosphatemia, and 68Ga-DOTATATE PET/CT helped to locate the culprit lesion. The present case report has some drawbacks, however. First, the patient’s serum FGF23 levels were not measured before and after surgery. Second, neither FGF23 nor somatostatin receptors were detected in tumor tissues, although the PET/CT result will typically demonstrate high somatostatin receptor expression in tumor tissues. The two tests limited further confirmation of the TIO diagnosis. We hope that our experience of this case will increase clinicians’ awareness of TIO and help to accelerate the diagnosis of TIO in clinical practice.
Conclusions
TIO is a rare condition worldwide. Currently, most patients with TIO can be successfully treated by surgical and medical therapy. However, the delayed diagnosis of TIO impairs its timely treatment. Although TIO is a surgical disease, the present case evidences that the prompt and accurate diagnosis of TIO requires a multidisciplinary clinical, pathological, and radiological collaboration.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (grant No. 82204871, Dr. Kai Qin) and the Chinese Medicine Scientific Research Project of the Health Commission of Hubei Province (grant No. ZY2021Q024, Dr. Kai Qin).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2116/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2116/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2116/coif). KQ reports that this study was supported by National Natural Science Foundation of China (No. 82204871) and Chinese Medicine Scientific Research Project of the Health Commission of Hubei Province (No. ZY2021Q024). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Agus ZS. Oncogenic hypophosphatemic osteomalacia. Kidney Int 1983;24:113-23. [Crossref] [PubMed]
- Ryan EA, Reiss E. Oncogenous osteomalacia. Review of the world literature of 42 cases and report of two new cases. Am J Med 1984;77:501-12. [Crossref] [PubMed]
- Salassa RM, Jowsey J, Arnaud CD. Hypophosphatemic osteomalacia associated with "nonendocrine" tumors. N Engl J Med 1970;283:65-70. [Crossref] [PubMed]
- Tan TH, Chen EJ, Chew MT, et al. Extended Whole-body Ga-68 DOTATATE PET-CT in evaluating Tumour-Induced Osteomalacia: Case report and review of literature. Nucl Med Mol Imaging 2021;55:130-5. [Crossref] [PubMed]
- Jan de Beur SM, Miller PD, Weber TJ, et al. Burosumab for the Treatment of Tumor-Induced Osteomalacia. J Bone Miner Res 2021;36:627-35. [Crossref] [PubMed]
- Chong WH, Molinolo AA, Chen CC, et al. Tumor-induced osteomalacia. Endocr Relat Cancer 2011;18:R53-77. [Crossref] [PubMed]
- Laurent MR, De Schepper J, Trouet D, et al. Consensus Recommendations for the Diagnosis and Management of X-Linked Hypophosphatemia in Belgium. Front Endocrinol (Lausanne) 2021;12:641543. [Crossref] [PubMed]
- Houang M, Clarkson A, Sioson L, et al. Phosphaturic mesenchymal tumors show positive staining for somatostatin receptor 2A (SSTR2A). Hum Pathol 2013;44:2711-8. [Crossref] [PubMed]
- Jiang Y, Hou G, Cheng W. Performance of 68Ga-DOTA-SST PET/CT, octreoscan SPECT/CT and 18F-FDG PET/CT in the detection of culprit tumors causing osteomalacia: a meta-analysis. Nucl Med Commun 2020;41:370-6. [Crossref] [PubMed]
- Abrahamsen B, Smith CD, Minisola S. Epidemiology of Tumor-Induced Osteomalacia in Denmark. Calcif Tissue Int 2021;109:147-56. [Crossref] [PubMed]
- Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 2003;348:1656-63. [Crossref] [PubMed]
- Wan E, Marks J, Wagner T, et al. Oncogenic osteomalacia: diagnosis, localisation, and cure. Lancet Oncol 2018;19:e365. [Crossref] [PubMed]
- Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 2012;92:131-55. [Crossref] [PubMed]
- González G, Baudrand R, Sepúlveda MF, et al. Tumor-induced osteomalacia: experience from a South American academic center. Osteoporos Int 2017;28:2187-93. [Crossref] [PubMed]
- Reyes-Múgica M, Arnsmeier SL, Backeljauw PF, et al. Phosphaturic mesenchymal tumor-induced rickets. Pediatr Dev Pathol 2000;3:61-9. [Crossref] [PubMed]
- Dahir K, Zanchetta MB, Stanciu I, et al. Diagnosis and Management of Tumor-induced Osteomalacia: Perspectives From Clinical Experience. J Endocr Soc 2021;5:bvab099.
- Jerkovich F, Nuñez S, Mocarbel Y, et al. Burden of Disease in Patients With Tumor-Induced Osteomalacia. JBMR Plus 2020;5:e10436. [Crossref] [PubMed]
- Qari H, Hamao-Sakamoto A, Fuselier C, et al. Phosphaturic Mesenchymal Tumor: 2 New Oral Cases and Review of 53 Cases in the Head and Neck. Head Neck Pathol 2016;10:192-200. [Crossref] [PubMed]
- El-Maouche D, Sadowski SM, Papadakis GZ, et al. (68)Ga-DOTATATE for Tumor Localization in Tumor-Induced Osteomalacia. J Clin Endocrinol Metab 2016;101:3575-81. [Crossref] [PubMed]
- He Q, Zhang B, Zhang L, et al. Diagnostic efficiency of (68)Ga-DOTANOC PET/CT in patients with suspected tumour-induced osteomalacia. Eur Radiol 2021;31:2414-21. [Crossref] [PubMed]



