
Comparative analysis of pre- and postmenopausal endometrial cancer in 216 patients
Highlight box
Key findings
• Compared to premenopausal EC, postmenopausal EC is more malignant and has a lower survival rate.
What is known, and what is new?
• Previous studies revealed that the clinical manifestations and characteristics of EC in premenopausal and postmenopausal women largely differed.
• Our study demonstrated that patients with postmenopausal EC had worse tumor pathological gradings, more severe muscular invasion, and a higher rate of lymphatic metastasis.
What is the implication, and what should change now?
• Due to the worse prognosis, patients with postmenopausal EC have a greater need for postoperative radiotherapy.
Introduction
Endometrial carcinoma (EC) is one of the most common gynecological malignancies. With the growing incidence of obesity, metabolic syndrome, and age-related disorders, EC has become more prevalent (1). Worse still, the worldwide cases of EC among the young population are also increasing (2). Ultrasonography is commonly applied for the initial screening of EC, while magnetic resonance imaging (MRI) is regarded as the best approach for preoperative pathological staging (3). Abnormal vaginal bleeding is the earliest symptom of EC. Obermair et al. (4) investigated 116 patients with postmenopausal EC and found that vaginal bleeding time was positively correlated with the degree of tumor malignancy. Surgery remains the principal therapeutic approach. The postoperative prognosis is largely affected by pathological factors, including the tumor pathological grade, deep-layer muscle invasion, lymphatic invasion, vascular invasion, and cervical invasion (5-8). Postoperative radiotherapy is the major adjunctive therapy for EC and can significantly reduce the recurrence of vaginal stump but fails to decrease distant recurrence or to improve the long-term survival rate of patients (9).
Previous studies have demonstrated that around 70% of cases of EC occur in postmenopausal women while 15% of cases occur in premenopausal women (10,11). The clinical manifestations and characteristics of EC in premenopausal and postmenopausal women differ and have distinct pathological stages and subtypes of EC. Nonetheless, there are still controversies concerning these 2 kinds of EC (12,13). In this study, we followed-up 216 patients with EC who were admitted to Wuhan Union hospital from August 2008 to August 2019 and conducted a controlled, retrospective study to examine the clinical characteristics of patients with EC. Consequently, we found that patients with premenopausal EC had better histological performance, less infiltration of the deep muscle layer, a lower malignant degree, a higher survival rate, and a better prognosis than did patients with postmenopausal EC. Compared to previous studies, our work included more clinical data and provided a comprehensive description of the key features of pre- and postmenopausal EC. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1616/rc).
Methods
A total of 216 patients with EC receiving surgical treatment in Wuhan Union hospital from August 2008 to August 2019 were enrolled in this study. The diagnosis of each EC case was further confirmed by a postoperative pathological examination. Patients were divided into 2 groups according to their menopause state: group A (the premenopausal group, accounting for 47.69%), which was composed of 103 patients aged from 28 to 62 years old (47.24±5.55 years old); and group B (the postmenopausal group, accounting for 52.31%), which was composed of 113 patients aged from 42 to 84 years old (57.70±7.01 years old). Participants were included if they satisfied the following criteria: (I) they were diagnosed with EC; (II) they had completed surgery in Wuhan Union hospital; (III) they had undergone a vaginal or abdominal ultrasound examination before surgery, including a pelvic computed tomography (CT) or MRI examination; (IV) they had complete general information; and (V) they were willing to cooperate with the follow-up investigations. Patients with artificial menopause were excluded.
Tissue specimens were obtained by hysteroscopy or diagnostic curettage before surgery and examined by histopathological staining. The pathological stage was evaluated according to the standard of the International Federation of Gynecology and Obstetrics for Endometrial Cancer (14). Naturally occurring menopause was defined as the absence of menstruation for over 12 months. Diagnosis of hypertension was based on the Chinese Hypertension Prevention and Treatment Guidelines (2018 edition) (15). Diagnosis of diabetes was based on the Chinese Diabetes Prevention and Treatment Guidelines (2016 edition) (16). Diagnosis of hyperlipidemia was based on the Chinese Adult Dyslipidemia Prevention and Treatment Guidelines (2016 edition) (17).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee, Tongji Medical College, Huazhong University of Science and Technology (No. 2021-S046), and individual consent for this retrospective analysis was waived.
Statistical analysis
SPSS 21.0 (IBM Corp, Armonk, NY, USA) was used for statistical analyses. For measurement data with a normal distribution, all parameters are expressed as the mean ± standard deviation (SD). Measurement data not conforming to the normal distribution are described as quartiles [median (interquartile range)]. The categorical variables are expressed as frequencies or proportions. The chi-squared test, Fisher exact probability test, or stratified chi-squared test were used for the univariate analysis of count data. The Student’s t-test or nonparametric rank sum test was applied for continuous data. A P value <0.05 was considered a statistically significant difference.
Results
Comparison of general clinical characteristics between the pre- and postmenopausal EC groups
There were 103 cases of premenopausal EC. The onset age was 28 to 62 years old, the median age was 48 years old, and the mean age was 47.24±5.55 years old. There were 113 cases of postmenopausal EC. The onset age was 42 to 84 years old, the median age was 56 years old, and the mean age was 57.70±7.01 years old. The age difference was statistically significant (P<0.05). Additionally, primary infertility was found in 9 (8.74%) premenopausal patients and 2 (1.77%) postmenopausal patients, and patients from premenopausal groups had fewer deliveries (median of post- and premenopausal groups: 2 vs. 1). As for the age of menarche, patients in the premenopausal group had earlier menarche compared with patients from the postmenopausal group (median age of post- and premenopausal groups: 14 years vs. 13 years).
Regarding the common comorbidities, incidences of diabetes (post- and premenopausal groups: 17.70% vs. 7.77%; P<0.05) and hyperlipemia (post- and premenopausal groups: 15.04% vs. 4.85%; P<0.05) were significantly higher in the postmenopausal EC group, while those of hypertension and liver dysfunction showed no difference. Moreover, the clinical manifestations of premenopausal patients with EC were mainly abnormal vaginal bleeding (80.58%) and menstrual changes. In comparison, the primary clinical manifestations of postmenopausal EC were abnormal vaginal bleeding (85.84%) and vaginal fluid (8.85%). Intriguingly, the anemia rate in premenopausal patients was higher than in the postmenopausal group (post- and premenopausal groups: 21.24% vs. 47.57%; P<0.05), which might result from chronic blood loss during a period of menstruation. Taken together, these data suggested that premenopausal and postmenopausal EC are largely different disease subtypes (Table 1).
Table 1
| Characteristics | Pre-EC (n=103) | Post-EC (n=113) | Statistical value | P value |
|---|---|---|---|---|
| Age1 (y), mean (SD) | 47.24 (5.55) | 57.70 (7.01) | 12.080 | <0.001 |
| Menarche2 (y), median [IQR] | 13 [12, 14] | 14 [13, 15] | –3.053 | 0.002 |
| Fertility4, n (%) | – | 0.0279 | ||
| Pregnable | 94 (91.26) | 111 (98.23) | ||
| Infertile | 9 (8.74) | 2 (1.77) | ||
| Pregnancy and delivery2 (n), median [IQR] | ||||
| Pregnancies | 3 [2, 4] | 3 [2, 4] | –1.245 | 0.213 |
| Deliveries | 1 [1, 2] | 2 [1, 2] | –2.726 | 0.006 |
| Familial tumor history3, n (%) | 2.649 | 0.104 | ||
| None | 90 (87.38) | 106 (93.81) | ||
| With | 13 (12.62) | 7 (6.19) | ||
| Hypertension3, n (%) | 0.179 | 0.672 | ||
| None | 80 (77.67) | 85 (75.22) | ||
| With | 23 (22.33) | 28 (2.48) | ||
| Diabetes3, n (%) | 4.711 | 0.030 | ||
| None | 95 (92.23) | 93 (82.30) | ||
| With | 8 (7.77) | 20 (17.70) | ||
| Hyperlipidemia4, n (%) | – | 0.014 | ||
| None | 98 (95.15) | 96 (84.96) | ||
| With | 5 (4.85) | 17 (15.04) | ||
| Liver function3, n (%) | 0.768 | 0.381 | ||
| Normal | 72 (69.90) | 85 (75.22) | ||
| Abnormal | 31 (30.10) | 28 (24.78) | ||
| Anemia3, n (%) | 16.701 | <0.001 | ||
| None | 54 (52.43) | 89 (78.76) | ||
| With | 49 (47.57) | 24 (21.24) | ||
| BMI1 (kg/m2), mean (SD) | 24.43 (3.36) | 23.94 (3.61) | 1.035 | 0.302 |
| Obesity3, n (%) | 3.231 | 0.072 | ||
| None | 43 (41.75) | 61 (53.98) | ||
| With | 60 (58.25) | 52 (46.02) | ||
| Manifestations4, n (%) | 8.310 | 0.040 | ||
| Vaginal bleeding | 83 (80.58) | 97 (85.84) | ||
| Vaginal fluid | 5 (4.85) | 10 (8.85) | ||
| Abdominal pain | 5 (4.85) | 0 (0.00) | ||
| Others | 10 (9.71) | 6 (5.31) | ||
| Hospital stay2 (d), median [IQR] | 16 [14, 19] | 18 [15, 21] | –2.668 | 0.008 |
1, Student t-test; 2, nonparametric U test; 3, chi-squared test; 4, Fisher exact test. y, years; SD, standard deviation; IQR, interquartile range; n, number; d, days; BMI, body mass index; EC, endometrial carcinoma.
Comparison of intraoperative conditions and pathological stages between the pre- and postmenopausal EC groups
The duration of surgery, amount of bleeding, and dissected lymph nodes were nearly equal between groups, indicating that all participants had been subjected to similar surgical conditions. Regarding the pathological type, both premenopausal and postmenopausal EC primarily manifested as endometrioid adenocarcinoma (post- and premenopausal groups: 91.26% vs. 91.15%; P=0.977) with similar pathological staging and tumor sizes. The muscular invasion, cervix uterine invasion, and periuterine invasion were all comparable between the groups. However, the histological grade of premenopausal EC was predominantly G1 (67.96%), whereas a mixed G1+G2 (42.48% and 32.74%, respectively) phenotype was observed in patients with postmenopausal EC. Consistently, compared to the premenopausal group, the incidences of vascular invasion (post- and premenopausal groups: 10.62% vs. 1.94%; P<0.05) and lymphatic metastasis (post- and premenopausal groups: 10.62% vs. 0.97%; P<0.01) were also higher in the postmenopausal EC group. Altogether, these data implied that EC occurring at the postmenopausal stage is generally more aggressive (Table 2).
Table 2
| Variables | Pre-EC (n=103) | Post-EC (n=113) | Statistical value | P value |
|---|---|---|---|---|
| Operation time1 (h), median [IQR] | 3 [3, 4] | 3 [3, 4] | –1.773 | 0.076 |
| Blood loss1 (mL), median [IQR] | 200 [150, 300] | 200 [150, 300] | –1.695 | 0.090 |
| Removed lymph nodes1, median [IQR] | 32 [19, 39] | 28 [18, 36] | –1.294 | 0.196 |
| Pathology2, n (%) | 0.001 | 0.977 | ||
| Adenocarcinoma | 94 (91.26) | 103 (91.15) | ||
| Non-adenocarcinoma | 9 (8.74) | 10 (8.85) | ||
| Staging3, n (%) | – | 0.288 | ||
| I | 85 (82.52) | 84 (74.34) | ||
| II | 10 (9.71) | 11 (9.73) | ||
| III | 7 (6.80) | 17 (15.04) | ||
| IV | 1 (0.97) | 1 (0.88) | ||
| Grading3, n (%) | – | 0.001 | ||
| G1 | 70 (67.96) | 48 (42.48) | ||
| G2 | 20 (19.42) | 37 (32.74) | ||
| G3 | 12 (11.65) | 26 (23.01) | ||
| G4 | 1 (0.97) | 2 (1.77) | ||
| Muscular invasion2, n (%) | 3.087 | 0.079 | ||
| None or <1/2 layers | 72 (69.90) | 66 (58.41) | ||
| ≥1/2 layers | 31 (30.10) | 47 (41.59) | ||
| Vascular invasion3, n (%) | – | 0.012 | ||
| None | 101 (98.06) | 101 (89.38) | ||
| With | 2 (1.94) | 12 (10.62) | ||
| Cervical invasion2, n (%) | 0.042 | 0.837 | ||
| None | 94 (91.26) | 104 (92.04) | ||
| With | 9 (8.74) | 9 (7.96) | ||
| Affecting the uterine adnexa3, n (%) | – | 0.293 | ||
| No | 98 (95.15) | 103 (91.15) | ||
| Yes | 5 (4.85) | 10 (8.85) | ||
| Lymphatic metastasis3, n (%) | – | 0.003 | ||
| No | 102 (99.03) | 101 (89.38) | ||
| Yes | 1 (0.97) | 12 (10.62) | ||
| Para-uterine invasion3, n (%) | – | 0.248 | ||
| No | 103 (100.00) | 110 (97.35) | ||
| Yes | 0 (0.00) | 3 (2.65) | ||
| Tumor size2, n (%) | 0.262 | 0.609 | ||
| Max diameter <3 cm | 64 (62.14) | 74 (65.49) | ||
| Max diameter ≥3 cm | 39 (37.86) | 39 (35.51) |
1, nonparametric U test; 2, chi-squared test; 3, Fisher exact test. IQR, interquartile range; n, number; EC, endometrial carcinoma.
Comparison of the postoperative prognosis between the pre- and postmenopausal EC groups
After surgical treatment, a low and comparable incidence of thrombus formation was detected in both groups (post- and premenopausal groups: 7.08% vs. 8.74%; P=0.651). Although the requirement for chemotherapy showed no significant difference (post- and premenopausal groups: 55.75% vs. 60.19%; P=0.509), a greater portion of patients from the postmenopausal EC group required additional radiotherapy (post- and premenopausal groups: 8.85% vs. 0.97%; P<0.05). The survival rate was also much lower in the postmenopausal EC group (post- and premenopausal groups: 81.19% vs. 95.15%; P<0.01), indicating that postmenopausal EC had a worse postoperative prognosis (Table 3).
Table 3
| Variables | Pre-EC (n=103) | Post-EC (n=113) | Statistical value | P value |
|---|---|---|---|---|
| Thrombosis1, n (%) | 0.204 | 0.651 | ||
| No | 94 (91.26) | 105 (92.92) | ||
| Yes | 9 (8.74) | 8 (7.08) | ||
| Chemotherapy1, n (%) | 0.436 | 0.509 | ||
| No | 62 (60.19) | 63 (55.75) | ||
| Yes | 41 (39.81) | 50 (44.25) | ||
| Radiotherapy2, n (%) | – | 0.011 | ||
| No | 102 (99.03) | 103 (91.15) | ||
| Yes | 1 (0.97) | 10 (8.85) | ||
| Survival2, n (%) | – | 0.008 | ||
| Alive | 98 (95.15) | 94 (81.19) | ||
| Dead | 5 (4.85) | 19 (16.81) |
1, chi-squared test; 2, Fisher exact test. n, number; EC, endometrial carcinoma.
Survival analysis of the pre- and postmenopausal EC groups
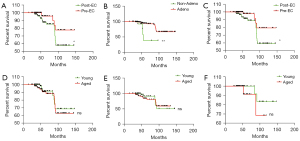
As expected, patients from the postmenopausal group had a lower survival rate (P<0.05; Figure 1A), which was in accordance with the prognostic data that suggested that postmenopausal EC had a higher mortality rate than did premenopausal EC. By classifying patients into adenocarcinoma and nonadenocarcinoma groups, we found that patients who did not have adenocarcinoma had a lower survival rate (Figure 1B). Both premenopausal and postmenopausal EC primarily manifested as endometrioid adenocarcinoma (Table 2). We further identified a similar worse survival in patients with postmenopausal EC diagnosed with adenocarcinoma (Figure 1C). Interestingly, with the division of patients into a young group (the first 50-year interval) and an aged group (the last 50-year interval), we found no significant difference in the recruited patients (Figure 1D-1F) except for a slight association in the premenopausal EC group of older age with worse prognosis (Figure 1F).
Discussion
The proactive molecular risk classification tool for endometrial cancers (ProMisE) has classified EC into 4 molecular subtypes: ultra-mutated EC with somatic mutations in DNA replicase polymerase ɛ (best prognosis), microsatellite instability-high (MSI-H) EC with a defect in the mismatch repair (MMR) pathway, copy number low EC with a wild-type TP53 gene, and copy-number-high EC with TP53 mutations (18). Such molecular typing is largely based on genomic characterization and thus has certain limitations in clinical practice. Recently, studies have endeavored to clarify the immune landscape of EC (19). By performing gene set variation analysis (GSVA) enrichment analysis to cluster The Cancer Genome Atlas EC samples, 4 immune subtypes of EC were defined: C1 (immunodepression), with the lowest T helper cell (Th)1:Th2 ratio and the poorest immunologic activity; C2 [interferon γ (IFN-γ) dominant], with a higher presence of IFN-γ as well as lymphocyte and macrophage infiltration; C3, with the highest proportion of Th17 cells and the strongest immunologic activity; and C4 (immunologically balanced), with a state of balanced immune condition (20). The advancements in the understanding of the immune microenvironment and the genetic profiling of EC can help in the development of novel therapeutics. Apart from the gold standard taxane-platinum combinatorial therapy, the efficacy of immune checkpoint inhibitors such as anti-programmed cell death protein 1/programmed death-ligand 1 (anti-PD-1/PD-L1), and antiangiogenic agents, such as lenvatinib has been demonstrated for EC treatment (21).
Although EC can occur at any age, 70–75% of cases develop after menopause (22). In this study, premenopausal patients accounted for 47.69% of the participants, higher than what was expected (23-25). This finding suggests an increasing prevalence of EC among the younger population, which may be related to environmental pollution, dietary change, late marriage and parturition, the use of contraceptives, hormone replacement therapy, and the advancement of early diagnostic techniques (26). We also found that premenopausal patients were associated with an earlier age of menarche, a higher incidence of infertility, and fewer pregnancies and deliveries. The absence of pregnancy or delivery is a known risk factor of EC, given that elevated progesterone exhibits an antagonistic effect on estrogen (22).
Obesity is another risk factor involved in the development and progression of EC. It is reported that more than 70% of patients with early EC are obese (27). In China, the obesity rate is as high as 42% in patients with EC (28). Lines of evidence suggest that, due to the decreased level of sex hormone-binding protein, plasma-free estradiol is increased in patients with obesity, leading to endometrial lesions via the long-term effect of estrogen. A prospective study conducted by Renehan et al. (29) found that an increment in BMI of 5 kg/m2 significantly elevated the risk of EC [relative risk (RR): 1.59]. Additionally, the RR for a BMI between 30 and 34.9 kg/m2 was 2.53, which dramatically increased to 6.25 when the BMI reached 40 kg/m2. In our study, obesity accounted for 58.25% of patients in the postmenopausal group and 46.02% of patients in the premenopausal group. There was no statistical difference between the 2 groups, which was consistent with the results presented by Zhu et al. (30). We reasoned that, even though EC is a wasting disease that could cause weight loss, obesity remains a major pathogenic factor affecting both EC subtypes.
Apart from obesity, diabetes has also been closely linked to the occurrence of EC by a number of studies (31). Diabetes increases the risk of EC, and the risk is further heightened in patients with obesity and diabetes (32). Researchers have proposed that insulin resistance–induced hormone imbalance is a major causal mechanism, which can be effectively alleviated by exercise, dietary intervention, and drug therapy (32). Diabetes-associated dyslipidemia has also drawn much attention. In 2014, China released the expert consensus on the management of dyslipidemia in postmenopausal women (33). In our study, the proportion of premenopausal patients with diabetes and hyperlipidemia was lower than that of the postmenopausal group, suggesting that diabetes and hyperlipidemia were more frequently observed after menopause. In the clinic, we should strengthen the awareness of managing cardiovascular disease risk in postmenopausal patients, especially those with diabetes and hyperlipidemia.
Postmenopausal patients mostly have special pathological types characterized by insufficient differentiation state, deep muscular infiltration, and early lymph node metastasis, resulting in a low 5-year survival rate (34). We also found that the prognosis of patients from the postmenopausal EC group was poorer. Among the 216 patients that were followed up, 19 died in the postmenopausal group (n=113) while 5 died in the premenopausal group (n=103), with the difference being statistically significant. Pelvic and abdominal lymph node metastasis is the most important factor affecting EC prognosis (35). The results of our study were in accordance with this finding: premenopausal patients with lymphatic metastasis accounted for 0.97%, while the proportion of postmenopausal patients with EC and with lymphatic metastasis was 10.62%. The difference might also be attributed to age, since age is positively correlated with the histological type of EC, and the prognosis of older adult patients is generally poor (36). This was also the case in our study, as the mean age was 47.24±5.55 years old in the premenopausal patients and 57.70±7.01 years old in the postmenopausal patients.
Conclusions
The pathological characteristics of premenopausal and postmenopausal EC are largely different. Premenopausal EC has a better histological phenotype, less infiltration of the deep muscle layer, a lower degree of malignancy, a higher survival rate, and a better prognosis than does postmenopausal EC. Nonetheless, there are limitations to the current study. First, this study was a retrospective analysis performed on patients with EC from one medical center. The sample size was relatively small, and the conclusions drawn should be further verified by large-cohort, multicenter, clinical trials. Second, our study only analyzed the traditional clinicopathological features of EC. Whether there are differences in hormone receptor status, DNA ploidy, and oncogene expression between postmenopausal and premenopausal EC needs to be determined by further investigation.
Acknowledgments
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1616/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1616/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1616/coif). YLL received grants from the Natural Science Foundation of Hubei Province (No. 2021CFB589). FS received grants from the National Natural Science Foundation of China (No. 82200923). JYL received grants from the National Natural Science Foundation of China (No. 82104488). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee, Tongji Medical College, Huazhong University of Science and Technology (No. 2021-S046), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet 2016;387:1094-108. [Crossref] [PubMed]
- Duska LR, Garrett A, Rueda BR, et al. Endometrial cancer in women 40 years old or younger. Gynecol Oncol 2001;83:388-93. [Crossref] [PubMed]
- Makker V, MacKay H, Ray-Coquard I, et al. Endometrial cancer. Nat Rev Dis Primers 2021;7:88. [Crossref] [PubMed]
- Obermair A, Hanzal E, Schreiner-Frech I, et al. Influence of delayed diagnosis on established prognostic factors in endometrial cancer. Anticancer Res 1996;16:947-9. [PubMed]
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 2013;31:2607-18. [Crossref] [PubMed]
- Ni J, Zhu T, Zhao L, et al. Metabolic syndrome is an independent prognostic factor for endometrial adenocarcinoma. Clin Transl Oncol 2015;17:835-9. [Crossref] [PubMed]
- Yoshiba T, Takei Y, Machida S, et al. Prognosis of endometrial cancer patients with and without symptoms at recurrence. J Obstet Gynaecol Res 2016;42:1814-21. [Crossref] [PubMed]
- Binder PS, Mutch DG. Update on prognostic markers for endometrial cancer. Womens Health (Lond) 2014;10:277-88. [Crossref] [PubMed]
- Yang M. Clinical analysis of 144 cases of premenopausal and postmenopausal endometrial carcinoma. Wenzhou: Wenzhou Medical University, 2014.
- Fang YH, Zhou YW, Yan SJ. Endometrial carcinoma in premenopausal and postmenopausal patients: an analysis of 166 cases. Anhui Medical and Pharmaceutical Journal 2013;17:2058-60.
- Jin LJ, Zhang YW, Wei YZ. Clinical characteristics of endometrial cancer patients. Hebei Medical Journal 2014;36:874-6.
- Liu H, Jian FF, Song W, et al. Clinical and pathological characteristics of endometrial carcinoma in premenopausal and postmenopausal patients. Journal of Shanghai Jiaotong University 2017;37:1670-3. (Medical Science).
- Huang SF, Li XM, Li T. Endometrial carcinoma in premenopausal and postmenopausal patients. Chinese General Practice 2008;11:2028-9.
- Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet 2009;105:109. [Crossref] [PubMed]
- Chinese guidelines for the management of hypertension (2018 revision). Chinese Journal of Cardiovascular Medicine 2019;24:24-56.
- Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition). Chin J Practical Internal Med 2018;38:292-344.
- Zhu JR, Gao RL, Zhao SP, et al. Guidelines for prevention and control of dyslipidemia in adults in China (revised in 2016). Chinese Circulation Journal 2016;31:937-53.
- Genestie C, Leary A, Devouassoux M, et al. Histological and molecular classification of endometrial carcinoma and therapeutical implications. Bull Cancer 2017;104:1001-12. Erratum in: Bull Cancer 2018 Feb;105(2):214. [Crossref] [PubMed]
- Antomarchi J, Ambrosetti D, Cohen C, et al. Immunosuppressive Tumor Microenvironment Status and Histological Grading of Endometrial Carcinoma. Cancer Microenviron 2019;12:169-79. [Crossref] [PubMed]
- Li BL, Wan XP. Prognostic significance of immune landscape in tumour microenvironment of endometrial cancer. J Cell Mol Med 2020;24:7767-77. [Crossref] [PubMed]
- Rousset-Rouviere S, Rochigneux P, Chrétien AS, et al. Endometrial Carcinoma: Immune Microenvironment and Emerging Treatments in Immuno-Oncology. Biomedicines 2021;9:632. [Crossref] [PubMed]
- Zhang M. Clinic pathological Study of 195 patients of Endometrial Carcinoma with Premenopausal and Post-Menopausal Women. Dalian: Dalian Medical University, 2013.
- Tran BN, Connell PP, Waggoner S, et al. Characteristics and outcome of endometrial carcinoma patients age 45 years and younger. Am J Clin Oncol 2000;23:476-80. [Crossref] [PubMed]
- Duong LM, Wilson RJ, Ajani UA, et al. Trends in endometrial cancer incidence rates in the United States, 1999-2006. J Womens Health (Larchmt) 2011;20:1157-63. [Crossref] [PubMed]
- Gao Y, Zhao M, Dai X, et al. The prevalence of endometrial cancer in pre- and postmenopausal Chinese women. Menopause 2016;23:884-7. [Crossref] [PubMed]
- Yu X, Chen B, Xie X, et al. Analysis of clinical and pathological changes of endometrial carcinoma. Journal of Practical Oncology 2004;19:244-5.
- Setiawan VW, Pike MC, Karageorgi S, et al. Age at last birth in relation to risk of endometrial cancer: pooled analysis in the epidemiology of endometrial cancer consortium. Am J Epidemiol 2012;176:269-78. [Crossref] [PubMed]
- Iemura A, Douchi T, Yamamoto S, et al. Body fat distribution as a risk factor of endometrial cancer. J Obstet Gynaecol Res 2000;26:421-5. [Crossref] [PubMed]
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. [Crossref] [PubMed]
- Zhu HY, Lai AL. Clinical analysis of 120 cases of endometrial carcinoma in premenopausal and postmenopausal women. Chinese Journal of Medicine 2007;42:37-9.
- von Gruenigen VE, Waggoner SE, Frasure HE, et al. Lifestyle challenges in endometrial cancer survivorship. Obstet Gynecol 2011;117:93-100. [Crossref] [PubMed]
- Weiderpass E, Persson I, Adami HO, et al. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden). Cancer Causes Control 2000;11:185-92. [Crossref] [PubMed]
- Liu HL, Hu DY, Han YL, et al. Chinese experts' consensus on the management of dyslipidemia in postmenopausal women. Chinese Journal of Cardiology 2014;42:279-83. [PubMed]
- Ren R, Jiang JY. Clinical and pathological characteristics of postmenopausal endometrial carcinoma. Maternal and Child Health Care of China 2007;13:1847-49.
- Li QQ, Ni GT. Research progress of lymph nodes in the treatment of early endometrial cancer. Anhui Journal of Preventive Medicine 2014;35:254-6.
- Jolly S, Vargas CE, Kumar T, et al. The impact of age on long-term outcome in patients with endometrial cancer treated with postoperative radiation. Gynecol Oncol 2006;103:87-93. [Crossref] [PubMed]


