
Pyrotinib for HER2-positive metastatic breast cancer: a systematic review and meta-analysis
Introduction
According to the World Health Organization’s (WHO) 2020 Global Cancer Statistics Report, there are approximately 2.3 million newly diagnosed breast cancer patients worldwide, accounting for 11.7% of the overall new cancer cases, which has surpassed lung cancer as the most frequent malignant tumor (1). Breast cancer has become an increasingly severe disease burden that threatens women’s health (2). Approximately 15% to 20% of breast cancers are overexpressed with human epidermal growth factor receptor 2 (HER2), which are more biologically aggressive, less responsive to chemotherapy, and have higher rates of recurrence and metastasis (3). Researchers have produced several medications that specifically target HER2, with a combination of trastuzumab and pertuzumab being widely utilized in clinical practice as first-line therapy, significantly improving the prognosis of patients with HER2-positive breast cancer. Nevertheless, patients will inevitably develop resistance to anti-HER2-targeting agents and relapse. As a result, the continual examination of relevant resistance mechanisms and the development of novel anti-HER2-targeted medications are crucial.
Pyrotinib, an irreversible tyrosine kinase inhibitor (TKI) that is independently developed in China, can inhibit the HER1, HER2, and HER4 families. The efficacy and tolerability of Pyrotinib have been demonstrated in clinical trials in comparison to lapatinib, an approved reversible HER2 TKI (4,5). Additionally, Pyrotinib administered with brain irradiation exhibited a superior therapeutic impact on brain metastases in patients with metastatic breast cancer (6). Since its listing in 2018 in China, Pyrotinib has been broadly applied in clinical practice as a recommended second-line treatment for metastatic breast cancer in developing nations lacking access to innovative antibody-drug conjugates (ADCs) such as ado-trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd) and different trials have been conducted to prove its efficacy and safety. Therefore, to determine the efficacy and safety of Pyrotinib in treating patients with HER2-positive metastatic breast cancer, we performed this systematic review and meta-analysis. We present the following article in accordance with the MOOSE checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1746/rc).
Methods
Search strategy
This meta-analysis research protocol was submitted and registered on the INPLASY platform, registration number: INPLASY202230076. When writing this systematic review and meta-analysis, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed. PubMed, Embase, Web of Science, and the Cochrane Library were searched with “Pyrotinib” as the major search phrases. The retrieval date is up to February 2022. Table S1 illustrates the detailed search strategy for the literature.
Criteria for study selection
Literature was assessed for inclusion and exclusion criteria by two separately. The inclusion criteria were: (I) patients with HER2-positive metastatic breast cancer; (II) previously treated with Pyrotinib in any line of therapy; (III) prospective or retrospective clinical trials, and (IV) single- or dual-arm studies. The exclusion criteria were: (I) review, letter comments or case reports; (II) in vitro experiments, animal studies; (III) other irrelevant research; (IV) clinical trials in the neoadjuvant phase; (V) ongoing clinical trials; (VI) duplicate publications in different journals.
Data extraction and quality assessment
Data were collected independently by two data extractors. The extracted basic information included author, year, study type, sample size, median age, treatment regimen, treatment line, doses of Pyrotinib, etc. The primary outcome endpoints extracted were overall objective response rate (ORR), progression-free survival (PFS) and safety data. Secondary endpoint data were the ORR rate in patients previously treatmed with lapatinib or trastuzumab/T-DM1 and those with brain metastases.
The literature included in the analysis was assessed with appropriate methods and tools: the Cochrane collaboration’s tool for assessing the risk of bias was used to evaluate randomized controlled trials (RCTs), the Newcastle-Ottawa Scale (NOS) was used to assess cohort studies and case-control studies, and the MINORS tool was used to evaluate single-arm studies, the Agency for Healthcare Research and Quality (AHRQ) was used to assess real-world studies.
Statistical analysis
R software (version 4.0.3) was used for performing this meta-analysis. The means of single-group descriptive statistics were combined and proportionate meta-analyses were performed to evaluate weighted pooled incidence rates dependent on the amount of diagnostically assessable patients. For dichotomous variables, with reference to the magnitude of heterogeneity, a fixed-effect model or a random-effects model was chosen to combine the effect sizes. The I2 statistic refers to the proportion of observed between-study variation (observed due to true heterogeneity rather than chance). The I2 statistic was calculated to assess study heterogeneity (7), I2 values of 25%, 50% and 75% were categorized as low, medium and high heterogeneity, respectively (8). Random-effects models were used to estimate heterogeneous effect sizes for each trial if P=0.10 or I2>50%; otherwise, a fixed-effects model was employed. Relative risks (RR) or odds ratios (OR) were used as pooled statistics to explain the statistical outcomes of several studies of dichotomous variables. The rate distribution must follow as nearly as feasible to the normal distribution due to the single-group rate data. In the case that the initial rate did not correspond to the normal distribution, the rate would need to be altered to conform to or approximate the normal distribution to increase the reliability of the combined results. The method of pooling was determined by the rate distribution. Pooled hazard ratios (HR) were calculated to compare PFS with Pyrotinib versus lapatinib, funnel plots and Egger’s test were then used to examine the results for publication bias. The major results were evaluated through sensitivity analysis, and heterogeneity was identified through subgroup analysis.
Results
Figure 1 demonstrates the flow chart for this study. By reviewing the titles and abstracts of 449 articles, 226 duplicates and 161 irrelevant articles were eliminated from the electronic database search result. Among the remaining publications, articles of interest were further reviewed, with twelve duplicate reports and twenty-seven ineligible articles excluded. A total of 23 studies were included, including three randomized controlled trials (RCT) (4,5,9), six prospective trials (10-15), eight retrospective trials (16-23), and six real-world studies (6,24-28). The baseline characteristics of the studies included are summarized in Table 1. The methodological quality of the included studies was evaluated, and the results are presented in Table S2.
Table 1
| Study/year | Study design | Sample, size (n) | Median age (years) | Treatment | Treatment line | Pyrotinib dose (mg) | No. of BM | ORR | PFS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | BM | Overall | BM | ||||||||||
| Li et al. 2021 (6) | Real world | 218 | 51 [34–75] | Pyrotinib/+H/X/N/nab-p | 1–3 line | 400, 320, 240 | 53 | 96 | 23 | 9.3 (8.6–10.0) | 7.0 (6.1–7.8) | ||
| Sun et al. 2021 (24) | Real world | 65 | 50 [31–75] | Pyrotinib/+X/N/T | ≥2 line | 400, 320 | NR | 47 | 8 | NR | NR | ||
| Anwar et al. 2021 (25) | Real world | 168 | 50 [28–73] | Pyrotinib +H/X/T | All line | 400, 320 | 39 | 68 | NR | 8.0 (7.3–10.5) | 8.7 (6.4–11.9) | ||
| Li et al. 2019 (10) | Prospective | 28 | 48 [24–59] | Pyrotinib +X | 1-2 line | 160, 240, 320, 400 | NR | 22 | NR | 22.1 (9.0–26.2) | NR | ||
| Li et al. 2021 (16) | Retrospective | 97 | 53 [26–74] | Pyrotinib +N | 400, 320 | 23 | 33 | NR | 7.8 (4.7–10.8) | 6.3 (3.4–9.2) | |||
| Ma et al. 2017 (11) | Prospective | 36 | 47 [29–67] | Pyrotinib | 1-2 line | 80, 160, 240, 320, 400, 480 | NR | 18 | NR | 8.9 (5.8–10.0) | NR | ||
| Zhang et al. 2021 (12) | Prospective | 141 | 52 [29–78] | Pyrotinib +H/X/T | NR | 400, 320 | 21 | 27 | 7 | 12.0 (8.1–17.8) | 18.4 (5.5–18.8) | ||
| Ouyang et al. 2021 (26) | Real world | 94 | 49 [28–71] | Pyrotinib | 1-3 line | NR | NR | 38 | NR | NR | NR | ||
| Lin et al. 2020 (17) | Retrospective | 113 | 53 [24–84] | Pyrotinib/+H/X/N/T | All line | 400, 320, 240, 160 | 31 | 31 | NR | 6.3 (5.5–7.1) | 6.7 (4.7–8.7) | ||
| Song et al. 2020 (18) | Retrospective | 72 | 55 [32–79] | Pyrotinib +H/X/N/T/ET | All line | NR | 15 | 19 | NR | 7.6 (5.5–9.7) | 6.0 (2.2–9.8) | ||
| Yang et al. 2022 (19) | Retrospective | 31 | 56 [31–69] | Pyrotinib +H/X/N/T | ≥2 line | 400 | NR | 8 | NR | 4.5 (3.1–5.9) | 5.2 | ||
| Hua et al. 2020 (20) | Retrospective | 66 | NR | Pyrotinib | ≥2 line | NR | NR | 11 | NR | 6.4 (3.6–9.2) | NR | ||
| Yan et al. 2020 (21) | Retrospective | 52 | NR | Pyrotinib +X | NR | 400 | NR | 41 | 41 | NR | NR | ||
| Hao et al. 2021 (27) | Real world | 254 | 50 | Pyrotinib/+X/N/T | All line | NR | NR | 89 | NR | 11 | NR | ||
| Luo et al. 2021 (13) | Prospective | 113 | NR | NR | 1-3 line | NR | NR | 75 | NR | 14.1 | 15.2 | ||
| Yan et al. 2021 (14) | Prospective | 23 | NR | Pyrotinib + dalpiciclib | 1 line | 400 | NR | 15 | NR | NR | NR | ||
| Yan et al. 2021 (15) | Prospective | 78 | NR | Pyrotinib +X | NR | 400 | 78 | NR | 52 | NR | 12.1 (9.0–14.7) | ||
| Yang et al. 2021 (22) | Retrospective | 68 | 44 [33–55] | Pyrotinib +X/N/T/Others | ≥2 line | 320 | NR | 41 | NR | 9.0 | NR | ||
| 96 | Pyrotinib +X/N/T/Others | 33 | NR | 6.2 | NR | ||||||||
| Xie et al. 2021 (23) | Retrospective | 92 | 52 [26–74] | Pyrotinib +N | NR | 320, 400 | NR | NR | NR | 8.3 | NR | ||
| 132 | Lapatinib +X | NR | NR | 5.0 | NR | ||||||||
| Li et al. 2021 (28) | Real world | 55 | 47 [27–73] | Pyrotinib/+X/N/T/others | ≥2 line | 400 | NR | 9 | NR | 6.0 (4.7–7.3) | NR | ||
| 50 | T-DM1 | 10 | NR | 4.2 (3.6–4.8) | NR | ||||||||
| Ma et al. 2019 (4) | RCT | 65 | 48 [25–70] | Pyrotinib +X | 1–3 line | 400 | NR | 51 | NR | 18.1 (13.9–NR) | NR | ||
| 63 | Lapatinib +X | 36 | NR | 7.0 (5.6–9.8) | NR | ||||||||
| Xu et al. 2021 (5) | RCT | 134 | 50 [42–55] | Pyrotinib +X | 1–3 line | 400 | NR | 90 | NR | 12.5 (9.7–NR) | NR | ||
| 132 | Lapatinib +X | 68 | NR | 6.8 (5.4–8.1) | NR | ||||||||
| Jiang et al. 2019 (9) | RCT | 185 | NR | Pyrotinib +X | NR | 400 | NR | 127 | NR | 11.1 (9.7–16.5) | 6.9 (5.4-NR) | ||
| 94 | Placebo +X | 15 | NR | 4.1 (2.8–4.1) | 4.2 | ||||||||
ORR, objective response rate; PFS, progression-free survival; RCT, randomized controlled trial; BM, brain metastases; H, Trastuzumab; X, capecitabine; N, Vinorelbine; T, Taxane; nab-p, nab-paclitaxel; ET, endocrine therapy; NR, not report.
The ORR of Pyrotinib in HER2-positive metastatic breast cancer
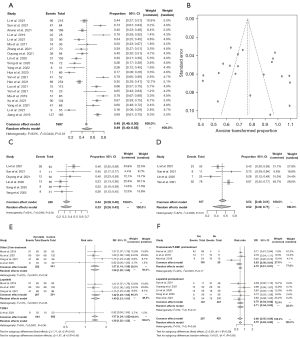
A total of 1,997 patients from twenty-one individual groups were included in the meta-analysis of ORR, with an overall ORR rate of 0.49 (95% CI: 0.40, 0.58), and the corresponding funnel plot of Egger’s test reveals publication bias (Egger’s test: t=0.42, P value =0.6757) (Figure 2A,2B, Figure S1). The result of the sensitivity analyses is shown in Figure S2. The overall ORR rate was 0.33 (95% CI: 0.24, 0.43) in patients previously treated with lapatinib, and 0.52 (95% CI: 0.32, 0.71) in brain metastatic patients (Figure 2C,2D). In ORR, Pyrotinib was superior to selected second-line drugs (RR =1.38, 95% CI: 1.25, 1.52) and lapatinib (RR =1.40, 95% CI: 1.21, 1.61), with significant statistical differences, but relatively inferior to T-DM1 (RR =0.82, 95% CI: 0.36, 1.85) (Figure 2E). Pyrotinib remained efficacious in patients pretreated with trastuzumab/T-DM1, with a relatively lower ORR rate than patients who had not previously received trastuzumab (RR =0.70, 95% CI: 0.61, 0.81); The ORR rate was lower in patients who had previously received lapatinib when compared with those who had not previously received lapatinib (RR =0.71, 95% CI: 0.54, 0.93) (Figure 2F).
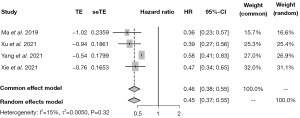
The survival outcome
Patients receiving Pyrotinib had a significantly longer PFS than those receiving lapatinib (HR =0.45, 95% CI: 0.37, 0.55) (Figure 3).
The safety analysis
Table 2 contains information on adverse reactions. The most common adverse reaction of any grade was diarrhea, with an incidence of 0.84 (95% CI: 0.74, 0.92), followed by nausea and vomiting 0.52 (95% CI: 0.36, 0.68), neutropenia 0.35 (95% CI: 0.23, 0.48), leukopenia 0.34 (95% CI: 0.23, 0.47), and palmoplantar erythema (PPE) 0.31 (95% CI: 0.16, 0.48). The most common adverse reaction of grade 3 or greater was diarrhea, with an incidence of 0.18 (95% CI: 0.15, 0.22), followed by PPE 0.07 (95% CI: 0.03, 0.11), neutropenia 0.05 (95% CI: 0.04, 0.07), and leukopenia 0.04 (95% CI: 0.02, 0.06).
Table 2
| Adverse reactions | Study/n | No. of people | ≥ Grade 3 | I2 | P | Study/n | No. of people | All grade | I2 | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhea | 18 | 1,841 | 0.18 (95% CI: 0.15, 0.22) | 75 | <0.01 | 13 | 1,026 | 0.84 (95% CI: 0.74, 0.92) | 93 | <0.01 |
| PPE | 14 | 1,561 | 0.07 (95% CI: 0.03, 0.11) | 87 | <0.01 | 12 | 995 | 0.31 (95% CI: 0.16, 0.48) | 97 | <0.01 |
| Neutropenia | 12 | 1,099 | 0.05 (95% CI: 0.04, 0.07) | 21 | 0.24 | 11 | 702 | 0.35 (95% CI: 0.23, 0.48) | 89 | <0.01 |
| Leukopenia | 11 | 1,160 | 0.04 (95% CI: 0.02, 0.06) | 70 | <0.01 | 9 | 702 | 0.34 (95% CI: 0.23, 0.47) | 90 | <0.01 |
| Thrombocytopenia | 9 | 873 | 0.00 (95% CI: 0.00, 0.01) | 46 | 0.06 | 6 | 415 | 0.09 (95% CI: 0.05, 0.15) | 60 | 0.03 |
| thrombocytopenia | 10 | 1,040 | 0.01 (95% CI: 0.00, 0.02) | 62 | <0.01 | 8 | 620 | 0.25 (95% CI: 0.15, 0.37) | 89 | <0.01 |
| Aminotransferase increased | 10 | 1,004 | 0.02 (95% CI: 0.01, 0.03) | 44 | 0.07 | 8 | 584 | 0.25 (95% CI: 0.14, 0.37) | 90 | <0.01 |
| Blood bilirubin increased | 8 | 842 | 0.01 (95% CI: 0.00, 0.02) | 62 | 0.01 | 6 | 481 | 0.27 (95% CI: 0.17, 0.39) | 86 | <0.01 |
| Rash | 9 | 1,020 | 0.00 (95% CI: 0.00, 0.01) | 41 | 0.09 | 8 | 697 | 0.11 (95% CI: 0.05, 0.21) | 84 | <0.01 |
| Nausea and vomiting | 12 | 1,195 | 0.03 (95% CI: 0.01, 0.05) | 73 | <0.01 | 11 | 888 | 0.52 (95% CI: 0.36, 0.68) | 96 | <0.01 |
| Fatigue | 8 | 745 | 0.00 (95% CI: 0.00, 0.01) | 27 | 0.22 | 5 | 287 | 0.14 (95% CI: 0.02, 0.33) | 92 | <0.01 |
| Dizziness | 5 | 636 | 0.00 (95% CI: 0.00, 0.01) | 73 | 0.01 | 5 | 426 | 0.05 (95% CI: 0.02, 0.10) | 71 | <0.01 |
| Oral mucositis | 7 | 1,017 | 0.01 (95% CI: 0.00, 0.02) | 56 | 0.04 | 5 | 428 | 0.11 (95% CI: 0.04, 0.19) | 84 | <0.01 |
| Blood creatinine increased | 3 | 226 | 0.00 (95% CI: 0.00, 0.00) | 0 | 1.00 | 4 | 264 | 0.16 (95% CI: 0.05, 0.32) | 91 | <0.01 |
| Decreased appetite | 5 | 558 | 0.01 (95% CI: 0.00, 0.03) | 53 | 0.08 | 6 | 596 | 0.17 (95% CI: 0.07, 0.30) | 93 | <0.01 |
PPE, palmar-plantar erythrodysesthesia.
Discussion
In recent years, ADCs such as T-DXd have made breakthroughs in the second-line treatment of breast cancer (29). However, since ADCs are currently unavailable in developing countries, Pyrotinib remains an inexpensive, safe, and effective therapeutic option. The efficacy and safety of Pyrotinib had been further established through persistent verification in clinical practice. Our study discovered an overall ORR rate of 0.49 (95% CI: 0.40, 0.58) for Pyrotinib in patients with HER2-positive metastatic breast cancer and 0.52 (95% CI: 0.32, 0.71) in those with brain metastases, indicating that Pyrotinib as a small-molecule HER-targeted drug has considerable efficacy in the second-line treatment of metastatic breast cancer and a definite effect on patients with brain metastases. One of the biggest challenges in treating breast cancer is its tendency to metastasize to other parts of the body, and after lung cancer, breast cancer is the second most common source of brain metastases (30). The most common site of metastasis in patients with HER2-positive breast cancer was found to be the brain (31). In studies that have demonstrated improved efficacy in patients with brain metastases, the majority include whole-brain radiotherapy (when multiple brain metastases are present) or local radiotherapy [stereotactic radiosurgery (SRS) when one or two brain metastases are present] in their treatment regimens to disrupt the blood-brain barrier, allowing Pyrotinib to reach the brain metastases. These treatments provide favorable clinical practice evidence for radiation therapy for brain metastases from breast cancer (32). After transtuzumab/T-DM1 or lapatinib therapy, it continues to work effectively, implying that Pyrotinib has the potential to reverse HER2 resistance. The latest basic experiments demonstrate that Pyrotinib combined with a novel CDK4/6 inhibitor SHR6390 can synergistically inhibit CDK4/6 and HER2 signaling pathways (33); a followed phase II research is underway to examine the efficacy of Pyrotinib in conjunction with the CDK4/6 inhibitor SHR6390 in patients with advanced HER2+/ER+ breast cancer who had previously received trastuzumab (34).
Due to the favorable anticancer activity and tolerability of Pyrotinib in combination with capecitabine in patients with recurrent or metastatic breast cancer that is positive for HER2, further trials of Pyrotinib in the neoadjuvant phase are also underway and have generated early findings. Xuhong et al. initially investigated the efficacy and safety of Pyrotinib in combination with epirubicin and cyclophosphamide, followed by docetaxel and trastuzumab in neoadjuvant treatment of stage I–III HER2-positive breast cancer, the pathological complete response rate in 19 patients was 73.7% (95% CI: 48.8–90.9) (35). The recently reported Panphila trial using TCbH combined with Pyrotinib (taxane + platinum + trastuzumab + Pyrotinib) neoadjuvant therapy achieved a pCR rate of 55.1% in 69 patients (36). Additionally, an ongoing neoadjuvant clinical trial compares the efficacy of Pyrotinib and pertuzumab when combined with trastuzumab plus nab-paclitaxel in the treatment of early or locally advanced HER2-positive breast cancer (37); another ongoing phase II trial is exploring the efficacy of Pyrotinib combined with neoadjuvant chemotherapy in patients with HR+/HER2-, HER4 overexpressing breast cancer (38). We are optimistic about Pyrotinib’s efficacy in the neoadjuvant treatment of HER2-positive breast cancer.
This meta-analysis has some limitations. Firstly, some analyses revealed significant heterogeneity. The possible explanations include discrepancies in study design, treatment line, disease assessment, and participant counts. Secondly, since individual patient data were not available, factual subgroup analyses were not performed (e.g., Estrogen receptor status, number of metastases, treatment lines, medication regimen, etc.). Thirdly, a subset of the survival data was extracted from the survival curves, which would culminate in error creation. Finally, since the majority of the studies were individual clinical trials, the evidence quality was weaker than meta-analyses of RCTs included.
Conclusions
The Pyrotinib-containing regimens exhibited satisfactory tumor response, disease control, and survival with tolerable adverse effects in patients with HER2-positive metastatic breast cancer receiving any line of therapy.
Acknowledgments
We would like to thank Professor Anhui Wang from the Military Preventive Medicine Teaching and Research Section of the Air Force Military Medical University, for his inspection and suggestions on the statistical aspects of this study are very significant to us.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1746/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1746/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Harbeck N, Gnant M. Breast cancer. Lancet 2017;389:1134-50. [Crossref] [PubMed]
- Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA 2019;321:288-300. [Crossref] [PubMed]
- Ma F, Ouyang Q, Li W, et al. Pyrotinib or Lapatinib Combined With Capecitabine in HER2-Positive Metastatic Breast Cancer With Prior Taxanes, Anthracyclines, and/or Trastuzumab: A Randomized, Phase II Study. J Clin Oncol 2019;37:2610-9. [Crossref] [PubMed]
- Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021;22:351-60. [Crossref] [PubMed]
- Li C, Bian X, Liu Z, et al. Effectiveness and safety of pyrotinib-based therapy in patients with HER2-positive metastatic breast cancer: A real-world retrospective study. Cancer Med 2021;10:8352-64. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Jiang ZF, Yan M, Hu XC, et al. Pyrotinib combined with capecitabine in women with HER2+metastatic breast cancer previously treated with trastuzumab and taxanes: A randomized phase III study. J Clin Oncol 2019;37:2. [Crossref]
- Li Q, Guan X, Chen S, et al. Safety, Efficacy, and Biomarker Analysis of Pyrotinib in Combination with Capecitabine in HER2-Positive Metastatic Breast Cancer Patients: A Phase I Clinical Trial. Clin Cancer Res 2019;25:5212-20. [Crossref] [PubMed]
- Ma F, Li Q, Chen S, et al. Phase I Study and Biomarker Analysis of Pyrotinib, a Novel Irreversible Pan-ErbB Receptor Tyrosine Kinase Inhibitor, in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J Clin Oncol 2017;35:3105-12. [Crossref] [PubMed]
- Zhang L, Wu X, Zhou J, et al. Pyrotinib in the Treatment of Women With HER2-Positive Advanced Breast Cancer: A Multicenter, Prospective, Real-World Study. Front Oncol 2021;11:699323. [Crossref] [PubMed]
- Luo T, Zhang Q, He P, et al. Real-world outcomes and safety of Pyrotinib in HER2-positive metastatic breast cancer (MBC) patients: A prospective cohort study. Ann Oncol 2021;32:S490. [Crossref]
- Yan M, Niu L, Lv H, et al. Dalpiciclib, a novel CDK4/6 inhibitor, combined with Pyrotinib for HER2+ advanced breast cancer: Interim results of a phase II trial. Ann Oncol 2021;32:S484. [Crossref]
- Yan M, Ouyang Q, Sun T, et al. Pyrotinib plus capecitabine for HER2-positive metastatic breast cancer patients with brain metastases (PERMEATE): A multicenter, single-arm phase II study. J Clin Oncol 2021;39. [Crossref]
- Li Y, Qiu Y, Li H, et al. Pyrotinib Combined With Vinorelbine in HER2-Positive Metastatic Breast Cancer: A Multicenter Retrospective Study. Front Oncol 2021;11:664429. [Crossref] [PubMed]
- Lin Y, Lin M, Zhang J, et al. Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis. Cancer Res Treat 2020;52:1059-66. [Crossref] [PubMed]
- Song GH, Li HP, Di LJ, et al. Efficacy and safety of oral Pyrotinib in HER2 positive metastatic breast cancer: real-world practice. Beijing Da Xue Xue Bao Yi Xue Ban 2020;52:254-60. [PubMed]
- Yang C, Shangguan C, Lou G, et al. The efficacy of pyrotinib-based therapy in lapatinib-resistant metastatic HER2-positive breast cancer. Ann Palliat Med 2022;11:332-8. [Crossref] [PubMed]
- Hua Y, Yang F, Yang Y, et al. 50P Efficacy and safety analysis of Pyrotinib in lapatinib resistant HER2-positive metastatic breast cancer: A retrospective study. Ann Oncol 2020;31:S1262. [Crossref]
- Yan M, Ouyang Q, Sun T, et al. Pyrotinib and capecitabine for HER2–positive metastatic breast cancer patients with previously untreated brain metastases: A single-group multicenter phase II study. Ann Oncol 2020;31:S360. [Crossref]
- Yang H, Wang W. Comparison of pyrotinib or lapatinib with chemotherapy for patients with HER2 positive breast cancer after first-line treatment failure: a retrospective study. Am J Transl Res 2021;13:10863-70. [PubMed]
- Xie Y, Li Y, Ting L, et al. Pyrotinib Plus Vinorelbine Versus Lapatinib Plus Capecitabine in Patients With Previously Treated HER2-Positive Metastatic Breast Cancer: A Multicenter, Retrospective Study. Front Oncol 2021;11:699333. [Crossref] [PubMed]
- Sun Y, Chen B, Li J, et al. Real-World Analysis of the Efficacy and Safety of a Novel Irreversible HER2 Tyrosine Kinase Inhibitor Pyrotinib in Patients with HER2-Positive Metastatic Breast Cancer. Cancer Manag Res 2021;13:7165-74. [Crossref] [PubMed]
- Anwar M, Chen Q, Ouyang D, et al. Pyrotinib Treatment in Patients With HER2-positive Metastatic Breast Cancer and Brain Metastasis: Exploratory Final Analysis of Real-World, Multicenter Data. Clin Cancer Res 2021;27:4634-41. [Crossref] [PubMed]
- Ouyang DJ, Chen QT, Anwar M, et al. The Efficacy of Pyrotinib as a Third- or Higher-Line Treatment in HER2-Positive Metastatic Breast Cancer Patients Exposed to Lapatinib Compared to Lapatinib-Naive Patients: A Real-World Study. Front Pharmacol 2021;12:682568. [Crossref] [PubMed]
- Hao L, Chen J, Chen M, et al. Real-world outcomes associated with Pyrotinib-based therapy for HER2-positive metastatic breast cancer. Ann Oncol 2021;32:S489. [Crossref]
- Li F, Xu F, Li J, et al. Pyrotinib versus trastuzumab emtansine for HER2-positive metastatic breast cancer after previous trastuzumab and lapatinib treatment: a real-world study. Ann Transl Med 2021;9:103. [Crossref] [PubMed]
- Trastuzumab Deruxtecan Data Impresses at ESMO. Cancer Discov 2021;11:2664-5.
- Shah N, Mohammad AS, Saralkar P, et al. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol Res 2018;132:47-68. [Crossref] [PubMed]
- Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003;97:2972-7. [Crossref] [PubMed]
- Mampre D, Mehkri Y, Rajkumar S, et al. Treatment of breast cancer brain metastases: radiotherapy and emerging preclinical approaches. Diagn Ther 2022;1:25-38. [Crossref] [PubMed]
- Wang Y, Yuan X, Li J, et al. The Synergistic Effects of SHR6390 Combined With Pyrotinib on HER2+/HR+ Breast Cancer. Front Cell Dev Biol 2021;9:785796. [Crossref] [PubMed]
- Nct. A Phase II Trial of Pyrotinib Combination With CDK4/6 Inhibitor SHR6390 in Patients Prior Trastuzumab-treated Advanced HER2-Positive Breast Cancer. Available online: https://clinicaltrialsgov/show/NCT04095390
- Xuhong J, Qi X, Tang P, et al. Neoadjuvant Pyrotinib plus Trastuzumab and Chemotherapy for Stage I-III HER2-Positive Breast Cancer: A Phase II Clinical Trial. Oncologist 2020;25:e1909-20. [Crossref] [PubMed]
- Liu Z, Wang C, Chen X, et al. Pathological response and predictive role of tumour-infiltrating lymphocytes in HER2-positive early breast cancer treated with neoadjuvant pyrotinib plus trastuzumab and chemotherapy (Panphila): a multicentre phase 2 trial. Eur J Cancer 2022;165:157-68. [Crossref] [PubMed]
- Nct. Pyrotinib Versus Pertuzumab in Combination With Neoadjuvant Trastuzumab and Nab-Paclitaxel in HER2+ Early or Locally Advanced Breast Cancer. Available online: https://clinicaltrialsgov/show/NCT04900311
- Nct. Pyrotinib in Combination With Neoadjuvant Chemotherapy in HR+/HER2-, HER4 High Expression Breast Cancer Patients: a Phase II Trial. Available online: https://clinicaltrialsgov/show/NCT04872985



