
Intraductal oncocytic papillary neoplasm (IOPN): two case reports and review of the literature
Highlight box
Key findings
• We describe two patients with IOPN—one accounting for the largest ever described—and provide a brief review of recent discoveries on the subject.
What is known and what is new?
• IOPNs are rare tumours affecting the pancreato-biliary system. Pancreatic IOPNs occur mainly locate in the head and proximal body and their average size is 5.5 cm. Pre-surgical differential diagnosis with IPMNs is challenging and often requires ancillary tests. IOPNs show little tendency to invade surrounding structures and metastasize.
• We describe a case of IOPN measuring 24 cm, the largest case ever described in literature. Despite its remarkable size, the tumour showed little aggressiveness, highlighting the biological peculiarities of IOPNs.
What is the implication, and what should change now?
• Given the rarity of IOPNs, further studies are required for a more precise radiological and pre-surgical identification of these neoplasms.
• A better understand of the biological mechanisms involved in the pathogenesis of these neoplasms is needed.
Introduction
Intraductal oncocytic papillary neoplasm (IOPNs) of the pancreas are infrequent tumours, accounting for 4.5% of intraductal pancreatic neoplasms (1). Firstly described in 1996 by Adsay et al. (2), these lesions have been recognized as distinct entities by the 2019 World Health Organization (WHO) classification of Digestive System Tumours. Along with intraductal papillary mucinous neoplasm (IPMN) and intraductal tubulopapillary neoplasm (ITPN), IOPNs belong to the category of intraductal pancreatic neoplasms.
Microscopically IOPNs are distinguished from IPMNs and ITPNs by the presence of dysplastic oncocytic elements arranged in frondous papillae.
Given the rarity of this neoplasm, we present two cases of IOPN as well as a review of the recent literature. The peculiarity of the IOPN described in case 1 is the extent of the size of the lesion, reaching a maximum of 24 cm. To the current day, this case accounts for the largest IOPN ever described in the literature. We present the following cases in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2029/rc).
Case presentation
Case 1
A 70-year-old Italian man presented to the general surgery department of our hospital in 2021, complaining of right abdominal pain radiating to the homolateral testicle. The patient had already been admitted to the emergency room for previous episodes of right renal colics. Ultrasound (US) revealed pyeloectasis and the presence of a large mass located in the mesogastric and anterior epigastric region, requiring additional abdominal computed tomography (CT).
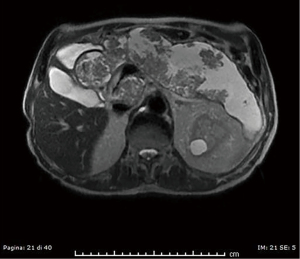
Abdominal CT revealed grade I hydronephrosis with mid-ureteral stone, a possible culprit for the referred symptoms. Pancreatic volume was consistently increased volume with loss of normal parenchymal lobulation. Magnetic resonance cholangiopancreatography (MRCP) highlighted multiple vegetations protruding into the main and accessory ducts (Figure 1). Contrast enhancement was also noted, in support of the proliferative nature of the lesion. The patient was scheduled for surgery, before which a second abdominal CT showed significant enlargement of the gland with involvement of the proximal part of the main pancreatic duct (MPD). Splenic, common hepatic, gastroduodenal arteries and portal confluence were not involved. A first diagnostic hypothesis was of ITPN.
Presurgical laboratory tests showed the following results: glucose level of 122 mg/dL [standard value (s.v.), 60–100 mg/dL], aspartate aminotransferase level of 17 IU/L (s.v., 2–31 IU/L), alanine aminotransferase level of 20 (s.v., 2–34 IU/L) and total bilirubin level of 0.23 mg/dL (s.v., 0.50–1.20 mg/dL). Serum level of carbohydrate antigen (CA)19-9 was 23 IU/mL (s.v., 0–27 IU/mL), of carcinoembryonic antigen (CEA) was 1.3 ng/mL (s.v., 0–5 ng/mL) and of CA125 was 11 IU/mL (s.v., 0–27 IU/mL).
The patient underwent total pancreatectomy with segmental duodenectomy and resection of the splenomesenteric portal venous confluence.
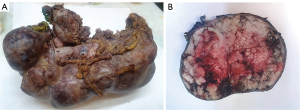
Gross examination of the pancreas revealed a 24×9×9 cm solido-cystic neoplasm entirely replacing the parenchyma, partially filled with mucin and with extensive hemorrhagic areas (Figure 2). Common bile duct presented a diameter of 1 cm.
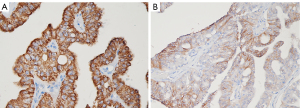
Histologically, the lesion was composed of intraductal papillary and cribriform structures lined by highly dysplastic oncocytic cells. The neoplastic elements showed wide, finely granular, eosinophilic cytoplasm and prominent nucleoli (Figure 3). Minimal (<5 mm) stromal infiltration was documented (Figure 4). Neither lymphatic nor vascular invasion was evident. On immunohistochemical (IHC), neoplastic cells were positive for cytokeratin 7, mucin (MUC)1, MUC5AC (Figure 5), MUC6 and HepPar1 (Figure 6A), focally positive for CD117(KIT) (Figure 6B) and negative for MUC2, CK20, Chromogranin A and Bcl-10. Collateral pancreatic parenchyma showed marked atrophy. Forty-eight regional lymph nodes were uninvolved showing reactive hyperplasia. A final diagnosis of IOPN with minimal stromal invasion was formulated, classified as pT1a pN0 R0 (3). In 10-month follow-up, the patient completely recovered and did not experience relapse.


Case 2
A 60-year-old Italian man was referred to the general surgery department of our hospital in 2022 with right lumbar pain radiating to the homolateral groin negative to the Giordano manoeuvre. US was not able to evidence the pancreatic gland due to marked meteorism. Clinical history was unremarkable.
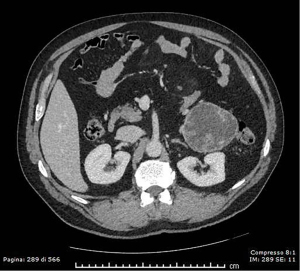
Abdominal contrast-enhanced CT revealed an 8.5×8×7.5 cm mass protruding from the pancreatic tail dislocating the left kidney and the descending colon. The lesion showed a hypodense central portion and 2 to 3 mm-thick walls lined by solid papillary-like projections with marked contrast-enhancement (Figure 7). A worrisome feature was the absence of a clear adipose cleavage plane with the left colic flexure. A preliminary diagnosis of degenerated IPMN of the terminal MPD was formulated.
Presurgical laboratory tests showed the following results: glucose level of 104 mg/dL (s.v., 60–100 mg/dL), aspartate aminotransferase level of 20 IU/L (s.v., 2–31 IU/L), alanine aminotransferase level of 42 (s.v., 2–34 IU/L) and total bilirubin level of 0.32 mg/dL (s.v., 0.50–1.20 mg/dL). Serum level determinations of CA19.9, CEA and CA125 were not available.
The patient underwent distal pancreatectomy with splenectomy two weeks after radiological identification.
Gross examination revealed a capsulated 8.5 cm × 8.5 cm × 8 cm mass located in the pancreatic body at 7.5 cm from surgical margin and communicating with the MPD. Cut surface was cystic and showed the presence of a mucinous content.
Microscopically, the lesion was composed of a papillary and cribriform proliferation involving secondary ducts. The epithelial component displayed variable grades of dysplasia and typical oncocytic features. A small focus (<5 mm) of stromal infiltration without lympho-vascular invasion was also noted. Forty regional lymph nodes demonstrated reactive hyperplasia.
IHC staining revealed positivity for cytokeratin 7, MUC5AC, MUC1 and MUC6; focal positivity for CD117 and HepPar1 and negativity for MUC2 and CK20. Collateral pancreatic parenchyma showed variable degrees of adipous involution.
The final diagnosis was determined to be IOPN with minimal stromal invasion, classified as pT1a N0 R0 (3).
After a 10-month follow-up, the patient has completely recovered and has not experienced relapse.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent were obtained from the patients for publication of these case reports and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
IOPNs of the pancreas are a relatively new entity within the family of intraductal papillary neoplasms. 2019 WHO classification of Digestive System Tumours raised IOPNs to a distinct nosological entity, in consideration of recent molecular and clinico-pathological data revealing the rather substantial differences between IOPNs and IPMNs.
Despite their rarity, a number of studies have been written on IOPNs since 1996 and a first systematic review by Wang et al. was published in 2019, collecting clinico-pathological data relative to 66 cases of IOPN and highlighting the need for large-scale cohort studies in order to strengthen our understanding of IOPNs (4).
In this case report, a brief review of the literature published from 2019 to date is presented. Only English written papers were included in the collection, impairing the statistical accuracy of data. A total of 80 IOPNs from 9 studies have been collected after the publication by Wang et al. clinico-pathological data for each case were not entirely available; a comprehensive list is available in Table 1.
Table 1
| Reference | No. of cases | Age (years) | Sex | Tumor size (mm) | Stromal invasion | Topography | LVI | PNI | LN status | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Wang (4) | 66 | n.a. | M:F =1:1 | 55 | 27/66 cases | n.a. | n.a. | n.a. | 6/55 | Alive without disease: 6/55 |
| Dead of disease: 4/50 | ||||||||||
| Dead of perioperative complications: 9/50 | ||||||||||
| Tanaka (5) | 3 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Hirabayashi (6) | 11 | 60.6 (mean) | n.a. | 45.5 (mean) | 9/11 cases | n.a. | n.a. | n.a. | n.a. | n.a. |
| Mattiolo (7) | 17 | n.a. | n.a. | n.a. | 2/17 cases | n.a. | n.a. | n.a. | n.a. | n.a. |
| Vyas (8) | 2 | 63 | M | 70 | Present | Head | n.a. | n.a. | n.a. | n.a. |
| 36 | M | 45 | Absent | Head | n.a. | n.a. | n.a. | n.a. | ||
| Kobayashi (9) | 6 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Singhi (10) | 20 | 43 | F | 34 | Absent | Body | n.a. | n.a. | n.a. | n.a. |
| 25 | M | 51 | Present | Body | n.a. | n.a. | n.a. | n.a. | ||
| 51 | F | 47 | Absent | Body | n.a. | n.a. | n.a. | n.a. | ||
| 67 | M | 45 | Absent | Head | n.a. | n.a. | n.a. | n.a. | ||
| 54 | M | 37 | Present | Head | n.a. | n.a. | n.a. | n.a. | ||
| 48 | F | 45 | Present | Body | n.a. | n.a. | n.a. | n.a. | ||
| 57 | M | 53 | Present | Tail | n.a. | n.a. | n.a. | n.a. | ||
| 64 | M | 70 | Present | Head | n.a. | n.a. | n.a. | n.a. | ||
| 27 | M | 45 | Absent | Body | n.a. | n.a. | n.a. | n.a. | ||
| 72 | M | 60 | Present | Head | n.a. | n.a. | n.a. | n.a. | ||
| 47 | M | 25 | Present | Head | n.a. | n.a. | n.a. | n.a. | ||
| 74 | M | 60 | Present | Head + body | n.a. | n.a. | n.a. | n.a. | ||
| 52 | F | 25 | Absent | Tail | n.a. | n.a. | n.a. | n.a. | ||
| 65 | M | 82 | Absent | Tail | n.a. | n.a. | n.a. | n.a. | ||
| 63 | M | 20 | Absent | Head | n.a. | n.a. | n.a. | n.a. | ||
| 64 | M | 60 | Present | Head | n.a. | n.a. | n.a. | n.a. | ||
| 57 | M | 35 | Absent | Head | n.a. | n.a. | n.a. | n.a. | ||
| 67 | M | 25 | Present | Tail | n.a. | n.a. | n.a. | n.a. | ||
| 50 | F | 65 | Present | Body | n.a. | n.a. | n.a. | n.a. | ||
| 75 | F | 51 | Absent | Head | n.a. | n.a. | n.a. | n.a. | ||
| Nakahodo (11) | 18 | 44 | M | 100 | Present | Body + tail | n.a. | n.a. | n.a. | n.a. |
| 45 | M | 15 | Present | Head | n.a. | n.a. | n.a. | n.a. | ||
| 47 | F | 40 | Present | Body + tail | n.a. | n.a. | n.a. | n.a. | ||
| 58 | F | 30 | Present | Head | n.a. | n.a. | n.a. | n.a. | ||
| 59 | F | 30 | Absent | Head | n.a. | n.a. | n.a. | n.a. | ||
| 61 | F | 25 | Absent | Body + tail | n.a. | n.a. | n.a. | n.a. | ||
| 61 | M | 35 | Absent | Head | n.a. | n.a. | n.a. | n.a. | ||
| 62 | M | 75 | Absent | Head | n.a. | n.a. | n.a. | n.a. | ||
| 64 | M | 12 | Absent | Body + tail | n.a. | n.a. | n.a. | n.a. | ||
| 67 | M | 30 | Present | Head | n.a. | n.a. | n.a. | n.a. | ||
| 69 | F | 22 | Absent | Head | n.a. | n.a. | n.a. | n.a. | ||
| 69 | F | 30 | Present | Head | n.a. | n.a. | n.a. | n.a. | ||
| 75 | M | 50 | Present | Body + tail | n.a. | n.a. | n.a. | n.a. | ||
| 76 | F | 70 | Present | Head | n.a. | n.a. | n.a. | n.a. | ||
| 79 | F | 50 | Absent | Body + tail | n.a. | n.a. | n.a. | n.a. | ||
| 80 | M | 22 | Absent | Head | n.a. | n.a. | n.a. | n.a. | ||
| 82 | F | 25 | Present | Body + tail | n.a. | n.a. | n.a. | n.a. | ||
| 82 | M | 26 | Absent | Body + tail | n.a. | n.a. | n.a. | n.a. | ||
| Innocenti (present study) | 2 | 75 | M | 240 | Present | Diffuse | Absent | Absent | N0 | Alive |
| 61 | M | 90 | Present | Tail | Absent | Absent | N0 | Alive |
IOPN, intraductal oncocytic papillary neoplasm; LVI, lymphovascular invasion; PNI, perineurial invasion; LN, lymph node; n.a., not available; M, male; F, female.
Age at presentation of IOPNs ranges from 36 to 84 years, occurring equally in males and females. Diagnosis is usually subsequent to unspecific abdominal symptoms or concurrent to imaging tests carried out for other reasons. Recurrent pancreatitis or painless jaundice can in rare case represent the onset manifestations. In a series of 182 pancreatic intraductal papillary neoplasms, including 3 IOPNs, Tanaka et al. found no correlation between oncocytic features and acute pancreatitis. On the other hand, intestinal and pancreato-biliary type IPMNs showed 22.4% and 35.7% incidence rates, respectively (5).
Available data collected from 2019 show that approximately half of the cases (22/43; 51.2%) were located in the head and proximal body and a slightly lesser number involved the distal body and tail (20/43; 46.5%).
Average size of IOPNs is 5.5 cm and tend to be slightly larger than IPMNs (1). The 54 cases collected from recent literature show a mean diametre of 4.8 cm.
The two cases collected from our institution fall within the expected age of diagnosis and show a prototypical clinico-pathological picture. In case 1 the diagnosis was subsequent to an unrelated renal colic, leading to the identification of the pancreatic lesion. Remarkably, at the time of diagnosis the normal pancreatic parenchyma was almost completely effaced by the florid IOPN growth. Diffuse involvement of the pancreas had already been described in the past, although it appears to be a rare circumstance.
CA19.9 and CEA serum levels are widely used in the preoperative setting of IPMNs. A rise in serum titres represents a worrisome feature suggestive of malignant transformation. Unlike IPMNs, IOPNs do not appear to be correlated with a rise in serological markers. Current preoperative tests are thus not useful for estimating the risk of degeneration. Case 1 was tested for tumour markers and CA19.9 and CEA levels were within the normality range.
Radiological diagnosis of IOPNs is exceptional, as both IPMNs and ITPNs appear as cystic dilations of the pancreatic ducts. Unlike IPMNs, ITPNs rarely display an evident cystic component and tend to present as solid masses filling the MPD (12). The presence of complex and arborizing papillae can represent a diagnostic hint for IOPN.
The high vascularity of IOPNs is responsible for the intense uptake of iodine and gadolinium-based contrast media, commonly interpreted as the presence of a solid component within the lesion.
The increased number of mitochondria inside oncocytes is accountable for the intense metabolic activity of oncocytic neoplasms. On positron emission tomography (PET) scan, IOPNs are characterized by a marked uptake of 18F-fluorodeoxyglucose (FDG) (13). To the unaware, high standardized uptake value (SUV) values could be inadvertently interpreted as sign of invasion, prompting to an aggressive surgical approach (14).
Fine-needle aspiration (FNA) has shown to be a powerful tool in the preoperative diagnosis of IOPN. A study from Reid et al. described 5 cases of IOPN preoperatively diagnosed via FNA; 4 out of 5 cases provided a hypercellular sample and 2 cases showed the presence of thick extracellular mucin (15).
Cytological differential diagnosis of IOPN focuses on oncocytic-like neoplasms such as oncocytic pancreatic neuroendocrine tumours (PanNETs), acinic cell carcinomas, ITPNs and metastatic carcinomas with oncocytic features.
Oncocytic PanNETs are aggressive variants characterized by large cells with eosinophilic cytoplasm, smooth nuclear contours and prominent eccentric nucleoli (16). IHC positivity for neuroendocrine markers rules out IOPNs.
Acinic neoplasms can cytologically resemble IOPNs, but the presence of brisk mitotic activity along with a lack of mucin and papillary structures point towards pancreatic acinic adenocarcinoma (15). Acinic differentiation can be confirmed via IHC with Bcl-10 and tripsin.
ITPNs pose a difficult differential diagnosis in consideration of their highly dysplastic appearance and presence of prominent nucleoli. Absence of the typical granular eosinophilic cytoplasm and almost complete absence of mucin are more suggestive of ITPN (17).
IOPNs grossly present under the form of complex solid-cystic masses, frequently located in the MPD. IOPNs rarely appear as purely cystic lesions and usually display a lesser degree of both extracellular and intracellular mucin production. On both macroscopic and microscopic grounds, mucin content appears to be a distinguishing feature between IOPNs and IPMNs. Enlarged MPD filled with mucus is a typical IPMN endoscopic finding and has not been reported in neither IOPNs nor ITPNs (18).
IOPNs are characterized by florid papillary projections protruding into the duct lumen, with variable coalescence into solid areas. The fibrovascular stalks of IOPN papillae are usually scant and can occasionally display areas of hyalinization. Hirabayashi et al. described the stromal characteristics of 11 cases of IOPN along with 168 cases of IPMN. The presence of hyalinised fibrovascular cores was much more commonly reported in IOPNs (45.5%) compared to IPMNs (1.2%). Hyalinized stroma showed IHC positivity for collagen and little to no positivity for laminin (6).
On IHC, IOPNs show diffuse positivity for MUC1 (EMA) and MUC6, similarly to ITPNs. MUC5AC and MUC2 are usually confined to the goblet cell population Oncocytic elements show a typical granular positivity for HepPar1.
Mattiolo et al. has investigated CD117 (c-KIT) expression in a cohort of 111 intraductal papillary neoplasms, including 17 IOPNs. CD117 positivity was able to distinguish IOPNs from all different IPMN subtypes, the former characterized by stronger and more diffuse pattern of expression (7).
Further, IHC peculiarities were noted by Basturk et al., such as higher rates of mesothelin and lower rates of claudin-4 expression if compared to IPMNs (19).
IOPNs display stromal invasion in up to 30% of cases (8,19). Out of the 71 available cases described from 2019 to date, 34 (47.9%) showed at least focal areas of stromal infiltration. Attention should be paid in distinguishing entrapped epithelial cells within tumour pseudocapsule from true stromal invasion.
Despite the presence of invasion, aggressive biological behaviour is uncommon. In a series of 55 IOPN cases described by Wang, 10 patients presented with either nodal, hepatic or cerebral metastases (4).
Marchegiani et al. described 18 cases of IOPN with specific focus to follow-up. Out of 18 patients, 6 displayed areas of overt invasive carcinoma. Recurrence rates at 5 and 10 years were 31% and 46% respectively, hinting at a possible role of field cancerization. Patients with evidence of recurrence undergoing complete pancreatectomy did not experience further relapses (20).
Genetic advances in the understanding of pancreatic papillary neoplasms reduced the ambiguity with regard to the nomenclature and the biology of such lesions.
More than 90% of IPMNs are known to harbour KRAS point mutations. Other commonly mutated genes are GNAS (40–60%), RNF43 (25%) and TP53 (10%) (21).
The genomic landscape of IOPNs substantially differs from that of IPMNs and displays a variety of alternative molecular prints. Basturk et al. demonstrated that none of their 22 IOPN cases harboured KRAS and GNAS mutations (19).
Kobayashi et al. extensively studied molecular pathways involved in the development of pancreatic papillary intraductal neoplasms; 2 of the 6 described IOPNs cases showed adenomatous polyposis coli (APC) mutations and presenting higher histological dysplasia if compared to APC wild-type IOPNs (9). Gastric, intestinal and pancreato-biliary IPMNs showed significantly lower rates of APC mutations.
The discovery of recurrent kinase gene rearrangements in IOPNs revived the interest in the biology of these entities.
Singhi et al. revealed the ubiquitous presence of fusion genes involving PRKACA and PRKACB sequences in a series of 20 IOPNs via RNA-targeted sequencing. None of the 3 described biliary IOPNs showed involvement of such genes, remarking the molecular differences between these lesions. Transcriptome sequencing identified 3 mutually exclusive fusion genes: ATP1B1-PRKACB, DNAJB1-PRKACA and ATP1B1-PRKACA recurring with a frequency of 50%, 30% and 20% respectively (10).
The increased transcription activity of DNAJB1 and ATP1B1 promoters elicit an over-expression of PRKACA and PRKACB sequences, ultimately leading increased protein kinase A (PKA) function.
In 2014, Honeyman et al. had already reported the presence of DNAJB1-PRKACA fusion in fibrolamellar hepatocarcinoma (22).
Vyas et al. also found DNAJB1-PRKACA and ATP1B1-PRKACA to be common mutations in pancreato-biliary IOPNs. According to the authors, the cytological similarities between fibrolamellar hepatocarcinoma and IOPNs could find their ground at a biochemical level, being PKA notoriously active on several substrates within the mitochondrial matrix. According to this hypothesis, the subsequent mitochondrial hyperplasia would be responsible of the oncocytic appearance of both tumour cell types (8).
Nakahodo et al. described over-expression of follistatin-coding mRNA in IOPNs. All of the 22 pancreato-biliary IOPNs showed IHC positivity for follistatin. Decreased apoptotic activity in IOPN cells—demonstrated by low expression of cleaved caspase 3 (CC3) on IHC—represents another interesting molecular finding (11).
Our understanding of IOPNs is growing but larger cohorts are needed in order to collect uniform data. Possible differences could lie between pure IOPNs and combined IOPNs and further studies will require to take this difference into consideration, especially if focusing on the biology of this entity.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2029/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2029/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2029/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent were obtained from the patients for publication of these case reports and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- D’Onofrio M, De Robertis R, Tinazzi Martini P, et al. Oncocytic Intraductal Papillary Mucinous Neoplasms of the Pancreas: Imaging and Histopathological Findings. Pancreas 2016;45:1233-42. [Crossref] [PubMed]
- Adsay NV, Adair CF, Heffess CS, et al. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol 1996;20:980-94. [Crossref] [PubMed]
- Amin MB, Edge SB, Greene FL, et al. editors. AJCC cancer staging manual. 8th edition. Chicago, IL, USA: Springer, 2017.
- Wang T, Askan G, Adsay V, et al. Intraductal Oncocytic Papillary Neoplasms: Clinical-Pathologic Characterization of 24 Cases, With An Emphasis on Associated Invasive Carcinomas. Am J Surg Pathol 2019;43:656-61. [Crossref] [PubMed]
- Tanaka T, Masuda A, Sofue K, et al. Acute pancreatitis in intraductal papillary mucinous neoplasms correlates with pancreatic volume and epithelial subtypes. Pancreatology 2021;21:138-43. [Crossref] [PubMed]
- Hirabayashi K, Kawanishi A, Morimachi M, et al. Hyalinized stroma is a characteristic feature of pancreatic intraductal oncocytic papillary neoplasm: An immunohistochemical study. Ann Diagn Pathol 2020;49:151639. [Crossref] [PubMed]
- Mattiolo P, Hong SM, Paolino G, et al. CD117 Is a Specific Marker of Intraductal Papillary Mucinous Neoplasms (IPMN) of the Pancreas, Oncocytic Subtype. Int J Mol Sci 2020;21:5794. [Crossref] [PubMed]
- Vyas M, Hechtman JF, Zhang Y, et al. DNAJB1-PRKACA fusions occur in oncocytic pancreatic and biliary neoplasms and are not specific for fibrolamellar hepatocellular carcinoma. Mod Pathol 2020;33:648-56. [Crossref] [PubMed]
- Kobayashi T, Omori Y, Ono Y, et al. Pathways for the development of multiple epithelial types of intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol 2021;56:581-92. [Crossref] [PubMed]
- Singhi AD, Wood LD, Parks E, et al. Recurrent Rearrangements in PRKACA and PRKACB in Intraductal Oncocytic Papillary Neoplasms of the Pancreas and Bile Duct. Gastroenterology 2020;158:573-82.e2. [Crossref] [PubMed]
- Nakahodo J, Fukumura Y, Saito T, et al. Upregulation of follistatin and low apoptotic activity in intraductal oncocytic papillary neoplasm of the pancreatobiliary system. Sci Rep 2020;10:8179. [Crossref] [PubMed]
- Zhang J, Ren S, Wang J, et al. Imaging findings of intraductal tubulopapillary neoplasm (ITPN) of the pancreas: Two case reports and literature review. Medicine (Baltimore) 2019;98:e14426. [Crossref] [PubMed]
- Noji T, Kondo S, Hirano S, et al. Intraductal oncocytic papillary neoplasm of the pancreas shows strong positivity on FDG-PET. Int J Gastrointest Cancer 2002;32:43-6. [Crossref] [PubMed]
- Fischer MA, Donati O, Heinrich S, et al. Intraductal oncocytic papillary neoplasm of the pancreas: a radio-pathological case study. JOP 2010;11:49-54. [PubMed]
- Reid MD. Cytologic Assessment of Cystic/Intraductal Lesions of the Pancreatobiliary Tract. Arch Pathol Lab Med 2022;146:280-97. [Crossref] [PubMed]
- Xue Y, Reid MD, Pehlivanoglu B, et al. Morphologic Variants of Pancreatic Neuroendocrine Tumors: Clinicopathologic Analysis and Prognostic Stratification. Endocr Pathol 2020;31:239-53. [Crossref] [PubMed]
- Tajima S. Intraductal tubulopapillary neoplasm of the pancreas suspected by endoscopic ultrasonography-fine-needle aspiration cytology: Report of a case confirmed by surgical specimen histology. Diagn Cytopathol 2015;43:1003-6. [Crossref] [PubMed]
- Reid MD, Stallworth CR, Lewis MM, et al. Cytopathologic diagnosis of oncocytic type intraductal papillary mucinous neoplasm: Criteria and clinical implications of accurate diagnosis. Cancer Cytopathol 2016;124:122-34. [Crossref] [PubMed]
- Basturk O, Chung SM, Hruban RH, et al. Distinct pathways of pathogenesis of intraductal oncocytic papillary neoplasms and intraductal papillary mucinous neoplasms of the pancreas. Virchows Arch 2016;469:523-32. [Crossref] [PubMed]
- Marchegiani G, Mino-Kenudson M, Ferrone CR, et al. Oncocytic-type intraductal papillary mucinous neoplasms: a unique malignant pancreatic tumor with good long-term prognosis. J Am Coll Surg 2015;220:839-44. [Crossref] [PubMed]
- Visani M, Acquaviva G, De Leo A, et al. Molecular alterations in pancreatic tumors. World J Gastroenterol 2021;27:2710-26. [Crossref] [PubMed]
- Honeyman JN, Simon EP, Robine N, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 2014;343:1010-4. [Crossref] [PubMed]



