
Clinicopathological analysis of primary thyroid non-Hodgkin lymphoma: a single-center study
Highlight box
Key findings
• The identification of immunoglobin clonal gene rearrangements is important for the differential diagnosis in patients with Hashimoto thyroiditis (HT) and a histologically benign lymphoepithelial lesion.
What is known and what is new?
• Primary thyroid lymphoma is quite rare and requires prompt diagnosis and treatment.
• We found that primary thyroid tumors were more common in middle-aged and older adult women with HT. Thyroid mucosa-associated lymphoid tissue (MALT) lymphomas can be misleading, and we emphasize the importance of gene rearrangement detection in the diagnosis of this tumor.
What is the implication, and what should change now?
• The routine detection of gene rearrangement should be used to inform the differential diagnosis in patients presenting with suspected HT.
Introduction
Primary thyroid lymphoma (PTL) is very rare, accounting for 1% to 8% of thyroid malignancies and 1% to 7% of all extranodal lymphomas (1,2). The most common type of PTL is diffuse large B-cell lymphoma (DLBCL), followed by mucosa-associated lymphoid tissue (MALT) lymphoma. Hashimoto thyroiditis (HT) is the only well-known risk factor for the development of PTL. Patients with HT are 40 to 80 times more at risk of developing lymphoma. However, only 0.6% of those with thyroiditis develop lymphoma (3,4). The prognosis is relatively good for PTLs, as most are indolent. However, relapse occurs in 6% to 26% of patients, always leading to poor outcomes. Since prognosis depends considerably on stages and subtypes, early diagnosis is important. Nevertheless, diagnosis of thyroid MALT lymphoma is often challenging, as it is nearly always confused with HT, thyroid medullary carcinoma, or undifferentiated carcinoma. Recently, the diagnosis of PTL has greatly improved through modern modalities, such as flow cytometry and immunohistochemical staining. In addition, ancillary studies including clonal immunoglobulin (Ig) gene rearrangements can provide important information for the determination of the classification and biological behaviors of lymphomas. These studies of primary nodal lymphomas have been well documented, but only a few sporadic reports have been published on PTL (5). We hypothesized that Ig gene rearrangement is critical for accurately diagnosing suspected PTL. Meanwhile, the management of PTL is still controversial, owing to its rarity and heterogenicity (6). Therefore, we undertook a retrospective study to describe the clinical characters, pathological features, and survival outcomes, and gene rearrangements of PTLs in our medical center. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2257/rc).
Methods
Patients and clinical criteria
Patients treated for PTL at our institution between 2007 and 2020 were retrospectively analyzed. PTL was staged according to the Ann Arbor staging classification as follows: stage IE, confined to the thyroid gland; stage IIE, spread beyond the thyroid to regional lymph nodes; stage IIIE, involvement of both sides of the diaphragm; and stage IVE, with disseminated disease. HT was diagnosed with the presence of pathological evidence combined with antithyroid antibodies and a clinical history of HT. Hepatitis B virus (HBV)-positive status included hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HbeAg), hepatitis B e antibody (HbeAb), or hepatitis B core antibody (HbcAb) marker positive, denoting infection with HBV or prior infection with HBV or inoculation with the hepatitis B vaccine.
The diagnosis of thyroid lymphoma was made based on histological examination in conjunction with the immunohistochemical results. PTL was defined as primary thyroid lymphoma with or without involvement of the local lymph nodes in the neck, and any other involvement beyond these limits occurring at the time of the first diagnosis was excluded. Clinical data were reviewed, including age, gender, diagnostic modality, the presence of HT, treatment, histopathologic types, and survival status. Patients with stage IE MALT underwent surgery or radiotherapy only, while patients with disease extending beyond the thyroid or with other disease types could either choose chemotherapy following surgery or combined modality treatment (CMT). All patients in the study were followed up, and their relevant was information recorded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (No. KY2021-077), and individual consent for this retrospective analysis was waived.
Pathological review
All cases were reviewed by 2 experienced pathologists. The specimens were subjected to histopathologic examination as well as immunohistochemistry analysis using a panel of monoclonal antibodies [cytokeratin (CK), thyroid transcription factor 1 (TTF1), CD20, CD19, CD3, CD5, CD21, CD10, B-cell lymphoma 2 protein (Bcl-2), B-cell lymphoma 6 protein (Bcl-6), MUM1, lambda, kappa (Maixin Biotech), Ki-67, and P53 (Leica)]. Fluorescent in situ hybridization (FISH) assays were carried out to detect the BCL2, BCL6, and MYC gene translocation in DLBCL lymphoma. The sections were deparaffinized, and the reaction was carried out using BCL2, BCL6, and the MYC with a dual-color break-apart FISH probe (Guangzhou LBP Medicine Science & Technology Co. Ltd., Guangzhou, China) according to the manufacturer’s instructions. Double-hit lymphoma (DHL) is recognized as “high grade B-cell lymphoma (HGBL) with rearrangements of MYC and BCL2 or BCL6”, while triple-hit lymphoma (THL) is defined as B-cell lymphoma with chromosomal alterations in MYC, BCL2, and BCL6 and has a similar prognosis to DHL (7).
Paraffin-embedded tissues were chosen for extracting genomic DNA and multiplex primer polymerase chain reaction (PCR) amplification was performed by using multiple groups of specific fluorescent primers. The PCR amplification products were analyzed using the, ABI 3500 genetic analyzer (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). The target genes for gene rearrangement detection in our research were selected using multiple groups of the following specific fluorescent primers: IGH VH-JH (FR-1,2,3, DH, DH7), IGK (Vκ-Jκ and Kde), and IGL (Vλ-Jλ). From each formalin-fixed paraffin-embedded (FFPE) tissue, 3-µm slices were dissected. DNA was extracted from the FFPE slices using the FFPE DNA extraction Kit (Amoy Diagnostics Co., Ltd., Xiamen, China), according to the manufacturer’s protocol. For the determination of monoclonality, sterile water was included as the negative sample and the target gene sequence plasmid DNA mixture (Shanghai Rigen Biotech Co., Ltd., Shanghai, China) as the positive sample. PCRs were carried out in a final volume of 20 µL consisting of 150 ng of genomic DNA, 16 µL of PCR solution, and 1 µL of DNA polymerase mixture. Amplification conditions followed the PCR thermocycler program, with initial preincubation at 95 ℃ for 7 minutes, followed by 40 cycles of 45 seconds at 95 ℃, 45 seconds at 60 ℃, and 90 seconds at 72 ℃. After the final cycle, an extension step was performed for 10 minutes at 72 ℃. After amplification, the PCR products were then renatured at 4 ℃. The ABI 3500 fluorescent genetic analyzer (Applied Biosystems) was used for the detection of clonal gene rearrangement in the amplified PCR products. The observation of more than 3 peaks in the valid size range, with the highest peak 3 times higher than the third highest peak, was considered to indicate monoclonality.
For quality control, 40 accurate quality control samples were tested, with a positive coincidence rate of 100%; 2 specific quality control samples were tested with a negative coincidence rate of 100%; 8 samples of precision quality control products were detected (via tests repeated 10 times) in the corresponding reaction solution; and 37 samples of limited quality control materials were detected. The kit could detect 10% of monoclonal Ig gene rearrangements against the background of 50 ng/µL of human genomic DNA. Clinical trials of 1,000 samples have been completed across 3 clinical trial institutions. Based on these trials, compared with the results of clinical diagnosis, this product has a sensitivity of 98.40% [95% confidence interval (CI): 96.55–99.26%], a specificity of 99.36% (95% CI: 98.36–99.75%), and total coincidence rate of 99.00% (95% CI: 98.16–99.45%). Monoclonal proliferation occurred when either tube was positive.
Statistical analysis
The survival curves were plotted according to the Kaplan-Meier method, and the log-rank test was performed to test the statistical significance. Statistical analyses were performed using SPSS v. 25.0 (IBM Corp., Armonk, NY, USA) statistical software. Disease-free survival (DFS) was defined as the time from treatment until relapse of lymphoma or death. Overall survival (OS) was defined as the time from diagnosis until death due to any cause. A value of P<0.05 was considered statistically significant.
Results
We collected the data of 22 patients diagnosed with PTL in our center. Of the 22 patients, 17 were female and 5 were male (female:male ratio, 3.4:1). The median age at diagnosis was 65.5 years (range, 44–80 years), and 11 (50.0%) patients were diagnosed with DLBCL lymphoma, 10 (45.5%) patients were diagnosed with MALT lymphoma, and 1 (4.5%) patient was diagnosed with T-cell lymphoma. A total of 17 (77.3%) patients were identified with HT. Among them, 3 (13.6%) had hypothyroidism before diagnosis. A total of 13 (59.1%) patients were positive for HBV. The distribution of the stage of disease according to the Ann Arbor staging system was as follows: stage I, 12 (54.5%) patients; stage II, 6 (27.3%) patients; stage III, 3 (13.6%) patients; and stage IV, 1 (4.5%) patient. Patients with stage III/IV were diagnosed with stage I/II at their first diagnosis, and then extension of the disease beyond the neck was revealed during their follow-up period. Among the 22 cases, 2 patients were diagnosed with PTL with operative fine needle aspiration (FNA) and underwent chemotherapy only, whereas the diagnosis was confirmed by thyroidectomy in the remaining 20 patients. Of these, 9 patients underwent surgery and chemotherapy and 11 patients underwent surgery only.
Morphologic features of MALT showed effacement of the normal architecture of the thyroid and formation of the lymphoepithelial lesion. Tumor cells showed diffuse positivity for CD20, which is a marker of B-cell lymphoma, but negativity for markers of cells that originated from the thyroid (Figure 1). Among the B-cell lymphoma cells, the lymphocytes were identified by immunohistochemistry as CD3−, CD 20+, CD79a+, CK–, and TTF1− cells. The T-cell lymphoma cells were identified as CD3+, CD20–, and CD79α– cells. In the B-cell lymphomas, 23.8% (5/21) of cells expressed CD10, 42.9% (9/21) expressed Bcl-6, 19.0% (4/21) expressed MUM1, and 28.6% (6/21) expressed Bcl-2, while the expression of the proliferation index, Ki-67, ranged from 8% to 90%. Neither double-hit nor THL was found in 8 of the DLBCL cases, and 2 cases of HT with lymphoid tissue dysplasia were subsequently diagnosed as MALT lymphoma by gene rearrangement testing (Figure 2).
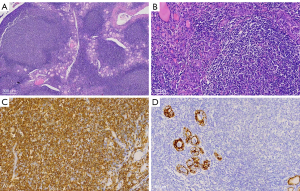
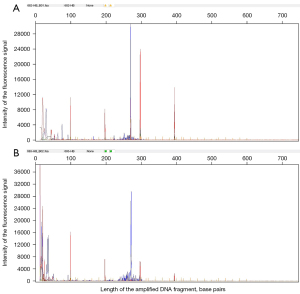
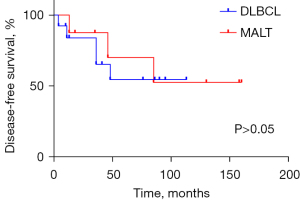
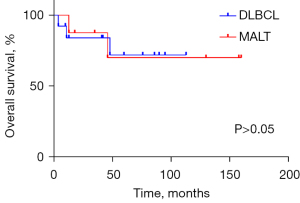
The clinical profiles of patients are presented in Table 1. One patient (No. 8: male, 65 years old, DLBCL) received 2 cycles of R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone) chemotherapy and then withdrew after developing a lung infection. He underwent second-line therapy [ICE (ifosfamide, carboplatin, and etoposide) + thalidomide] 5 months later, shifted to hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, adriamycin, dexamethasone) 2 months later, and eventually died of lung infection. The median follow-up time was 47 months (range, 4–160 months). In the study, 3 patients died from PTL, 2 patients died from other reasons (heart attack and pulmonary infection), and 4 patients suffered relapse (2 with neck nodes, 1 with mediastinal nodes, and 1 with neck and mediastinal nodes). There was no significant difference in the prognosis between the 2 subtypes of PTL in our study (Figures 3,4).
Table 1
| Case | Age (y) | Gender | Location | Stage | Subtype | HT | Hypothyroidism | HBV | Compression symptom | GCB/non-GCB | Ki-67 (%) | FISH | Treatment | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | F | Right | I | MALT | + | − | + | − | / | 8 | / | Operation 1 | 18, alive |
| 2 | 76 | F | Left | I | MALT | + | + | + | − | / | 30 | / | Operation 2 | 13, dead |
| 3 | 56 | F | Right | II | DLBCL | + | + | + | − | Non-GCB | 70 | (−) | Operation 3 + R-CHOP | 41, alive |
| 4 | 80 | M | Right | I | MALT | + | − | + | − | / | 20 | / | Operation 1 | 46, dead |
| 5 | 53 | F | Left | I | DLBCL | + | − | + | − | Non-GCB | 50 | (−) | Operation 2 | 60, relapse |
| 6 | 64 | M | Right | IV | DLBCL | + | − | − | − | Non-GCB | 50 | (−) | Operation 2 + CHOP | 76, alive |
| 7 | 67 | F | Right | II | DLBCL | − | − | + | − | Non-GCB | 90 | (−) | Operation 2 + R-CHOP | 90, alive |
| 8 | 65 | M | Right | II | DLBCL | + | − | − | − | GCB | 70 | (−) | R-CHOP-ICE + thalidomide-hyper-CVAD | 11, DOD |
| 9 | 64 | F | Left | II | DLBCL | + | − | / | − | Non-GCB | 60 | (−) | Operation1 + R-CHOP | 86, alive |
| 10 | 53 | F | Left | I | DLBCL | + | + | + | − | GCB | 60 | (−) | Operation 2 + R-CHOP | 95, alive |
| 11 | 56 | F | Left | III | DLBCL | − | − | / | − | Non-GCB | 90 | (−) | Operation 1 | 36, relapse |
| 12 | 64 | F | Right | I | MALT | + | − | + | − | / | 15 | / | Operation 2 + Rituximab 600 mg | 35, alive |
| 13 | 75 | F | Bilateral | II | MALT | + | − | − | − | / | 30 | / | Operation 2 | 85, relapse |
| 14 | 59 | F | Left | II | MALT | + | − | + | − | / | 30 | / | Operation 2 + R-CHOP | 130, alive |
| 15 | 61 | F | Left | I | MALT | + | − | − | − | / | 5 | / | Operation 1 | 160, alive |
| 16 | 72 | M | Bilateral | I | MALT | + | − | + | − | / | 20 | / | Operation 3 | 158, alive |
| 17 | 70 | F | Bilateral | III | DLBCL | + | − | + | + | GCB | 40 | / | Operation 2 + CHOP | 48, DOD |
| 18 | 72 | F | Left | I | DLBCL | − | − | + | − | GCB | 80 | / | CHOP | 113, alive |
| 19 | 71 | F | Right | III | DLBCL | − | − | − | + | GCB | 70 | / | Operation 1 + CHOP | 4, DOD |
| 20 | 68 | M | Bilateral | I | T cell | − | − | − | − | / | 20 | / | Operation 2 | 12, relapse |
| 21 | 66 | F | Left | I | MALT | + | − | + | − | / | 10 | / | Operation 2 | 13, alive |
| 22 | 44 | F | Left | I | MALT | + | − | − | − | / | 15 | / | Operation 2 | 10, alive |
“/” means the patients did not take the examinations. Operation 1: lobectomy; Operation 2: subtotal thyroidectomy; Operation 3: total thyroidectomy. DLBCL, diffuse large B-cell lymphoma; DOD, dead of disease; F, female; FISH, fluorescent in situ hybridization; GCB, germinal center B-cell; HBV, hepatitis B virus; HT, Hashimoto thyroiditis; hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone; ICE, ifosfamide, carboplatin, and etoposide; M, male; MALT, mucosa-associated lymphoid tissue; PTL, primary thyroid lymphoma; R-CHOP, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone; y, years.
Discussion
We undertook a retrospective study to describe the clinical characters, pathological features, and survival outcomes, and the clonal Ig gene rearrangements of PTLs in our medical center. As a result, we demonstrated that PTL affects middle-aged and older adult females with HT, with the main histological type being non-Hodgkin B-cell lymphoma, mostly DLBCL, followed by MALT, which is in accordance with the previous literature. Prognosis depends much on subtypes and stages, so early diagnosis is important. For patients with HT along with a histologically benign lymphoepithelial lesion that is suspicious for PTL, detecting clonal Ig gene rearrangements is essential for the differential diagnosis. Differences between DFS and OS between DLBCL and MALT were not statistically significant in our research.
Epidemiology of PTL
By definition, PTL involves only the thyroid gland with or without involvement of the local lymph nodes in the neck and without any other involvement beyond these limits at the time of the first diagnosis. The relevant research indicates that females are more likely to have PTL than are males, with a female-to-male ratio of 1.8–4.4:1, and usually in their fifth to eighth decades (2,8-12), which accords with our study. DLBCL is the most common subtype, accounting for 50% to 85% of cases, followed by MALT, accounting for 10% to 50% of cases, and the mixed subtype (a combination of DLBCL and MALT), accounting for 25% to 30% of cases (1,9,11). Other types, such as follicular lymphoma and T-cell lymphoma, are rare. The most important risk factor for PTL is HT, which causes a 40-to-80-fold increase in the risk of PTL (2,10). The prevalence of HT is 67.3% to 91.8% in patients with PTLs; the prevalence of HT is significantly higher in the MALT type compared with the DLBCL type and is also significantly higher in the DLBCL type with a MALT type component compared with the pure DLBCL type (13). Moreover, some authors have even suggested that all PTLs originate in an HT setting (14). In our study, a background of HT was identified in 17 of 22 (77.3%) cases, which concurs with relevant reports. The 3 cases without a pathological background of HT involved DLBCL and T-cell lymphoma, and in these cases, the tumor cells proliferated diffusely and destroyed the original thyroid structure. For 2 cases, only FNA samples were available, resulting in limited observable tissue, and therefore, the background of HT might not have been observable. Since HBV can infect B lymphocytes, the prevalence of lymphoma is higher in HB antigen-positive patients than in HB antigen-negative patients, especially for B-cell lymphoma (15,16).
Diagnostic modalities in PTL
The preoperative diagnosis of PTL is quite difficult, and PTL has been previously categorized as undifferentiated thyroid carcinoma. The most common clinical presentation of PTL is rapid enlargement of a mass in the neck which, in about 30% of patients, may cause compression symptoms such as dysphasia, dyspnea, coughing, and hoarseness (17), thus resembling anaplastic thyroid cancer (ATC). B-cell lymphoma symptoms, such as fever, night sweats, and weight loss, occur in 10–20% of patients (1,9), hyperthyroidism is present in 7–36% of patients, and HT occurs in 27–80.6% of patients (2,8,9,11,12), which is in accordance with our study.
Most commonly, ultrasound (US) of PTL shows a hypoechoic mass, with the echogenicity less than that of the adjacent neck musculature, combined with hypervascularity and a characteristically undifferentiated outline (17). Computed tomography (CT) and magnetic resonance imaging (MRI) show a homogenous mass with a lack of calcification, cystic degeneration, and necrosis. Typical imaging demonstrates homogenous, mild enhancement, and mild T2 hyperintensity compared with the surrounding thyroid gland (18). Currently, positron emission tomography (PET)-CT plays an important role in the detection and staging of PTL. PET-CT images show increased uptake of fluorodeoxyglucose (FDG) in the thyroid lobe, with/without lymph node involvement, and help to stage, restage, or evaluate the response to treatment. Bone marrow biopsy should also be performed to rule out marrow involvement. Noninvasive methods are recommended in the cases of a suspicious mass. FNA under US guidance can confirm the diagnosis for 70–80% of patients, but for low-grade lymphomas, especially when the distinction between MALT and HT is quite difficult, the distinguishing features may be the abundance of lymphoid tissue and a high proportion of intermediate centrocyte-like cells in low-grade PTL compared to HT (10). With the help of flow cytometry or immunohistochemical staining, the accuracy of FNA is increasing. Core or open surgical biopsy is still essential when the diagnose resulting from FNA is obscure or raises the suspicion of rare subtypes. Moreover, researchers still recommend that all patients undergo open excisional biopsy to make a definitive assessment of the exact subtype or stage of PTL. For most of the patients (20/22, 90.91%) in our study, PTL was accidentally discovered when the patients were undergoing surgical treatment, and this was possibly due to inadequate preoperative preparation or incomplete awareness of PTL.
Histopathological examination is the gold standard for the diagnosis of PTL. The tumor cells in DCBCL are 2 to 3 times larger than normal lymphocytes, which sometimes resemble epithelial cells and need to be differentiated from undifferentiated carcinoma by immunohistochemistry. CK and leukocyte common antigen (LCA) play an important role in differentiating between these 2 diagnoses. MALT lymphoma is part of a group of indolent, peripheral, B-cell lymphomas, originating from marginal zone cells (8). However, during the assessment of intraoperative frozen sections and routine pathological examinations, we often limit our attention to the presence of epithelial atypia and ignore the atypia of lymphoid tissue, especially when the hyperplasia is hidden against the pathological background of HT (8). In our study of tumors of the lymphatic hematopoietic system, the diagnosis of MALT lymphoma was often difficult because there were no specific immunohistochemical markers that could be used to clearly identify this type of tumor. A characteristic pathological feature was the presence of an interstitial infiltrate, which mainly consisted of lymphocytes organized into true lymphoid follicles. Gene rearrangement tests played a key role in diagnosis when we encountered confusion (5).
Gene rearrangement is a useful method for diagnosing malignant lymphocyte cloning. In our previous study, clonal relationships and identical light chain restrictions were found in DLBCL and MALT lymphoma in the stomach, suggesting that most DLBCLs derive from an evolution of MALT lymphomas (5). A similar hypothesis related to the thyroid is also supported by the relatively common finding of a MALT-type component coexistent with DLBCL (19). In contrast to gastric MALT lymphoma, primary MALT lymphoma of the thyroid has not been researched extensively. As for diagnosis, PCR-based analysis of Ig rearrangements has become the gold standard for the diagnosis of B-cell lymphoma over the past two decades (20). Most PTL presents as a monoclonal arrangement, while no arrangement is detected in HT (21), which helps to distinguish PTL from HT.
Therapy and prognosis of PTL
Treatment of PTL includes chemotherapy, surgery, and radiotherapy. DLBCL is regarded as a high-grade lymphoma with a more aggressive clinical course, and about 60% of patients are diagnosed at an advanced stage of disseminated disease (1,2). MALT is regarded as a low-grade lymphoma with a more indolent course. There has been a reduction in surgery since the development of new diagnostic modalities, as surgery may lead to surgical complications and the incomplete removal of residual tissue. Indolent subtype therapy involves local treatment with either surgery alone and/or local radiotherapy, while thyroidectomy alone or radiotherapy alone is only adequate for stage 1E MALT (6,17). A high-grade or progressive subtype usually requires a combination of chemotherapy and radiotherapy, referred to as CMT. CMT is reported to significantly reduce distant relapse and overall outcome (22). Radiotherapy doses range considerably from 12.5 to 45 Gy in different fractionation regimens. The most commonly used prescription ranges from 30 to 50 Gy (8,9,11). The involvement of the mediastinum as well as the neck in the treatment port has been emphasized in many studies (6,22,23). While radiation therapy can achieve local control of lesions, chemotherapy can solve distant dissemination of disease. There is a trend of applying chemotherapy in patients with PTL, including those with localized disease. CHOP therapy is widely used. Furthermore, there is a growing trend of administering rituximab, a monoclonal antibody that selectively binds to the CD20 antigen found on the surface of pre-B and mature B lymphocytes, with CHOP (R-CHOP treatment) for advanced patients. Relapse occurs in 6–26% of patients within an average time of 20–25.2 months (2,11), leading to poor outcomes (8). Second-line treatment, also called salvage therapy, is used in patients who have relapsed. Tracheostomy or tracheal stenting is performed if patients have airway obstruction. Tracheal stenting is an invasive, temporary palliation of airway compression.
PTL is staged according to the Ann Arbor staging classification, with imaging of the chest, abdomen, and pelvis, including bone marrow examination. Patients with HT and without “B” symptoms are found to have a significantly higher recurrence-free survival (RFS) without influence on the OS (9). Prognosis is very much dependent on the histologic subtype and stage, and the 5-year OS varies from 34.5% to 74% (2,6,11). MALT is associated with a good prognosis, and the 5-year OS is as high as 90–100%. This is followed by a 5-year OS of 78–100% for mixed MALT and diffuse large cell lymphoma, while DLBCL always leads to a poor prognosis, with a 5-year OS of 55–87.5% (1,6). According to the stages of disease, the 5-year OS of stage 1E without extrathyroidal invasion is 80%, that of stage 1E with extrathyroidal invasion is 58%, that of stage IIE is 50–53%, and that of stages IIIE and IVE is 36% (11,24). The 5-year OS of those with mediastinal lymph node involvement is significantly worse than it is in those without it (9). The 5-year RFS and OS for those patients who undergo operation alone (either partial or complete) are 77% and 87%, respectively (9). The International Prognostic Index (IPI) is significantly associated with OS , with OS being significantly higher in patients with an IPI of 0 than in those with an IPI of 1 (11). Older age, hoarseness, elevated serum urate, and stage III/IV lymphoma have a significant impact on prognosis (2). It appears that the abdominal cavity and gastrointestinal tract are the most common sites of failure in patients with non-Hodgkin lymphoma of the thyroid (11). It has been reported that patients with HBV infection respond poorly to chemotherapy than do those without HBV (16). A previous study showed that CD10, Bcl-6, MUM1, Bcl-2, or Ki-67 in 39 patients with PTL was not statistically correlated with OS (25). Meanwhile, patients may relapse about 10 years after their initial treatment, so long-term follow-up is needed (24).
Conclusions
PTL mainly affects female patients in their fifth and sixth decades, and the main histological type of PTL is non-Hodgkin B-cell lymphoma. A suspicious lump requires an FNA biopsy, and differentiation between thyroiditis and a thyroid solid tumor by means of flow cytometry or immunohistochemical staining is necessary. For patients with HT along with a histologically benign lymphoepithelial lesion, identification of clonal Ig gene rearrangements is important for routine differential diagnosis. There was no significant difference between the prognosis of DLBCL and MALT in our study, probably due to the small sample size. Further research of new diagnostic markers and therapeutic targets at the molecular level for effective management is needed.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2257/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2257/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2257/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2257/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (No. KY2021-077), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Widder S, Pasieka JL. Primary thyroid lymphomas. Curr Treat Options Oncol 2004;5:307-13. [Crossref] [PubMed]
- Pedersen RK, Pedersen NT. Primary non-Hodgkin's lymphoma of the thyroid gland: a population based study. Histopathology 1996;28:25-32. [Crossref] [PubMed]
- Noureldine SI, Tufano RP. Association of Hashimoto's thyroiditis and thyroid cancer. Curr Opin Oncol 2015;27:21-5. [Crossref] [PubMed]
- Walsh S, Lowery AJ, Evoy D, et al. Thyroid lymphoma: recent advances in diagnosis and optimal management strategies. Oncologist 2013;18:994-1003. [Crossref] [PubMed]
- Suzuki A, Hirokawa M, Higashiyama T, et al. Flow cytometric, gene rearrangement, and karyotypic analyses of 110 cases of primary thyroid lymphoma: a single-institutional experience in Japan. Endocr J 2019;66:1083-91. [Crossref] [PubMed]
- Pavlidis ET, Pavlidis TE. A Review of Primary Thyroid Lymphoma: Molecular Factors, Diagnosis and Management. J Invest Surg 2019;32:137-42. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Alzouebi M, Goepel JR, Horsman JM, et al. Primary thyroid lymphoma: the 40 year experience of a UK lymphoma treatment centre. Int J Oncol 2012;40:2075-80. [PubMed]
- Belal AA, Allam A, Kandil A, et al. Primary thyroid lymphoma: a retrospective analysis of prognostic factors and treatment outcome for localized intermediate and high grade lymphoma. Am J Clin Oncol 2001;24:299-305. [Crossref] [PubMed]
- Sakorafas GH, Kokkoris P, Farley DR. Primary thyroid lymphoma (correction of lympoma): diagnostic and therapeutic dilemmas. Surg Oncol 2010;19:e124-9. [Crossref] [PubMed]
- Ha CS, Shadle KM, Medeiros LJ, et al. Localized non-Hodgkin lymphoma involving the thyroid gland. Cancer 2001;91:629-35. [Crossref] [PubMed]
- Hirokawa M, Suzuki A, Hashimoto Y, et al. Prevalence and diagnostic challenges of thyroid lymphoma: a multi-institutional study in non-Western countries. Endocr J 2020;67:1085-91. [Crossref] [PubMed]
- Travaglino A, Pace M, Varricchio S, et al. Hashimoto Thyroiditis in Primary Thyroid Non-Hodgkin Lymphoma. Am J Clin Pathol 2020;153:156-64. [Crossref] [PubMed]
- Xia Y, Wang L, Jiang Y, et al. Sonographic appearance of primary thyroid lymphoma-preliminary experience. PLoS One 2014;9:e114080. [Crossref] [PubMed]
- Lemaitre M, Brice P, Frigeni M, et al. Hepatitis B virus-associated B-cell non-Hodgkin lymphoma in non-endemic areas in Western Europe: Clinical characteristics and prognosis. J Infect 2020;80:219-24. [Crossref] [PubMed]
- Wang Y, Wang H, Pan S, et al. Capable Infection of Hepatitis B Virus in Diffuse Large B-cell Lymphoma. J Cancer 2018;9:1575-81. [Crossref] [PubMed]
- Diaconescu MR, Costea I, Glod M, et al. An Unwonted Clinicopathological Subtype of Thyroid Primary Lymphoma. Chirurgia (Bucur) 2016;111:428-31. [Crossref] [PubMed]
- Aiken AH. Imaging of thyroid cancer. Semin Ultrasound CT MR 2012;33:138-49. [Crossref] [PubMed]
- Chai YJ, Hong JH. Clinicopathological characteristics and treatment outcomes of 38 cases of primary thyroid lymphoma: a multicenter study. Ann Surg Treat Res 2015;89:295-9. [Crossref] [PubMed]
- Langerak AW, Groenen PJ, Brüggemann M, et al. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia 2012;26:2159-71. [Crossref] [PubMed]
- Matsuzuka F, Fukata S, Kuma K, et al. Gene rearrangement of immunoglobulin as a marker of thyroid lymphoma. World J Surg 1998;22:558-61. [Crossref] [PubMed]
- Doria R, Jekel JF, Cooper DL. Thyroid lymphoma. The case for combined modality therapy. Cancer 1994;73:200-6. [Crossref] [PubMed]
- Harrington KJ, Michalaki VJ, Vini L, et al. Management of non-Hodgkin's lymphoma of the thyroid: the Royal Marsden Hospital experience. Br J Radiol 2005;78:405-10. [Crossref] [PubMed]
- Pyke CM, Grant CS, Habermann TM, et al. Non-Hodgkin's lymphoma of the thyroid: is more than biopsy necessary? World J Surg 1992;16:604-9; discussion 609-10. [Crossref] [PubMed]
- Bai Z, Li L, Guan T, et al. Clinical prognosis and bioinformatic analysis of primary thyroid lymphoma. Medicine (Baltimore) 2021;100:e24598. [Crossref] [PubMed]


