
Efficacy and safety of immune checkpoint inhibitors (ICIs) combined with antiangiogenic therapy for thymic epithelial tumors (TETs): a retrospective study
Highlight box
Key findings
• Immune checkpoint inhibitors (ICIs) with antiangiogenic therapy show considerable potential as a treatment option for thymic epithelial tumors (TETs).
What is known and what is new?
• ICIs combined with antiangiogenic therapy have shown marked efficacy against a range of advanced cancers. However, evidence is lacking as to whether this combination therapy could benefit TETs.
• The combination therapy yielded a median progression-free survival (PFS) of 6.7 months and a median overall survival (OS) of 45.6 months.
What is the implication, and what should change now?
• ICIs combined with antiangiogenic therapy may be employed as a treatment option for TETs. Further large-scale studies would be done to confirm whether or not the combination therapy is the best approach.
Introduction
Thymic epithelial tumors (TETs), which include thymomas (Tm) and thymic carcinomas (TC), are formed from thymic epithelial cells. According to the RARECARE project description, TETs are a type of rare malignancy in adults, with an annual incidence of 0.13 to 0.32 per 100,000 people (1). Patients with thymoma often present with autoimmune paraneoplastic syndromes, such as myasthenia gravis and pure red cell aplastic anemia. TC are more aggressive than Tm and are often diagnosed as stage IV (2).
For individuals with thymic malignancies, surgical resection is the best treatment option and the prognosis following surgery is generally good (3). After progression, however, the therapeutic options for advanced patients who have undergone first-line chemotherapy are limited. According to a number of clinical studies, immunotherapy targeting programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1) has exhibited moderate clinical effectiveness against TETs, but has been linked to severe immune toxicity, notably in Tm patients (4,5). Other therapeutic strategies, such as anti-angiogenesis therapy, present clinical benefits in the case of TETs (6-8). To improve treatment outcomes, immune checkpoint inhibitors (ICIs) coupled with antiangiogenic therapy have been examined in a wide range of advanced malignancies, and have presented encouraging anticancer efficacy (9-11). However, current research is lacking on the safety and efficacy of this combination of drugs against TETs.
Here, we retrospectively assessed the safety and efficacy of ICIs combined with antiangiogenic targeting agents as a chemotherapy-free regimen against TETs in a real-world setting. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2192/rc).
Methods
Patients
Thymic tumor patients who received ICIs combined with an antiangiogenic regimen in Zhejiang Cancer Hospital from April 2020 to May 2022 were included in our retrospective study. The main eligibility criteria for patients were: (I) aged 18 years or older, (II) histologically confirmed Tm or TC of clinical stage IV, and (III) an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. The main exclusion criterion was prior immune or antiangiogenic treatment. Clinical information for each of the patients, such as sex, age, smoking history, the ECOG PS, tumor histology, Masaoka stage, surgery history, prior lines of treatment, and sites of metastases, was retrieved from electronic medical records. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Committee at Zhejiang Cancer Hospital (No. IRB-2022-63) and individual consent for this retrospective analysis was waived.
Efficacy and safety
Data were collected from enrolled patients during disease progression. All patients were administered drug doses according to the National Comprehensive Cancer Network (NCCN) guidelines or clinical trials. Patients received treatment every two or three weeks with apatinib (250 mg) or anlotinib (12 mg) in combination with sintilimab (200 mg), nivolumab (3 mg/kg), or other PD-1 inhibitors until disease progression or unacceptable toxicity levels were observed. In a variety of contexts, including normal clinical care, extended access, and compassionate use programs, all enrolled patients received ICIs in conjunction with antiangiogenic targeted drugs.
Response Evaluation Criteria in Solid Tumors (RECIST,1.1) was used to assess the tumor response. The objective response rate (ORR) is the rate of the complete response (CR) plus the partial response (PR). The disease control rate (DCR) is the rate of ORR plus the stable response (SD). The period from the first administration of combination therapy to progressive disease (PD), death, or the initiation of other therapeutics was defined as progression-free survival (PFS). Overall survival (OS) was defined as the period from the initial diagnosis of advanced disease to death or the last follow-up. In the safety analysis, adverse events (AEs) were assessed using the U.S. National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Statistical analysis
Clinical data were analyzed using descriptive statistical methods. The median PFS and OS were determined by the Kaplan-Meier method and compared using the log-rank test, for both the total and subgroup values. We used SPSS version 25.0 and GraphPad prism version 9.0 to assess all of the statistical data. A P value of 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
We collected data for ten advanced Tm and TC patients who were undergoing, or had received, combination therapy with PD-1/PD-L1 inhibitors and an antiangiogenic regimen at Zhejiang Cancer Hospital. The majority of these patients were female (80%), with an ECOG PS of 1 (60%). The median age of the patients was 49 (range, 34–72). Two Tm and eight TC patients were included in the group, and the TC patients included five cases of squamous cell carcinoma, two cases of poorly differentiated carcinoma and one case of sarcomatoid carcinoma. The Masaoka stage was IVb (80% of patients) or stage IVa (20% of patients). Of the patients, 70% had not undergone surgery before receiving the combination therapy. Only one patient who received the combination regimen as the first-line setting, while the others received it as a multi-line setting. Tables 1,2 show the patient characteristics at baseline.
Table 1
| Characteristic | Values |
|---|---|
| Gender, n [%] | |
| Male | 2 [20] |
| Female | 8 [80] |
| Age (years) | |
| Median (range) | 49 (34–72) |
| ≥60, n [%] | 2 [20] |
| <60, n [%] | 8 [80] |
| Smoking history, n [%] | |
| Current/former | 2 [20] |
| Never | 8 [80] |
| ECOG PS, n [%] | |
| 0 | 4 [40] |
| 1 | 6 [60] |
| Histology, n [%] | |
| Thymic carcinoma | 8 [80] |
| Thymoma | 2 [20] |
| Masaoka’s stage, n [%] | |
| IVa | 2 [20] |
| IVb | 8 [80] |
| History of surgery, n [%] | |
| Yes | 3 [30] |
| No | 7 [70] |
| Number of prior therapy lines, n [%] | |
| First-line | 1 [10] |
| ≥second-line | 9 [90] |
ECOG PS, Eastern Cooperative Oncology Group performance status.
Table 2
| Patient number | Gender/age (years) | Smoking | Surgery | Histology | Stage | Metastatic site | Agent of combination strategy | Lines of therapy | Best tumor response |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female/34 | No | No | Squamous cell carcinoma | IVa | Pleura | PD-1 + apatinib/anlotinib | 2 | SD |
| 2 | Female/50 | No | No | Squamous cell carcinoma | IVb | Liver | PD-1 + apatinib | 2 | SD |
| 3 | Female/47 | No | Yes | Type AB thymoma | IVa | Pleura | PD-1 + apatinib | 1 | PR |
| 4 | Female/35 | No | No | Poorly differentiated carcinoma | IVb | Liver, bone | PD-1 + apatinib | 3 | SD |
| 5 | Female/60 | No | No | Poorly differentiated carcinoma | IVb | Pleura, lung | PD-1 + apatinib | 4 | PR |
| 6 | Male/47 | Yes | Yes | Sarcomatoid carcinoma | IVb | Pleura, bone | PD-1 + anlotinib | 2 | SD |
| 7 | Female/49 | No | No | Thymoma | IVb | Bone | PD-1 + apatinib | 3 | SD |
| 8 | Female/53 | No | No | Squamous cell carcinoma | IVb | Lung, liver | PD-1+ lenvatinib | 5 | SD |
| 9 | Male/72 | Yes | No | Squamous cell carcinoma | IVb | Liver | PD-1 + anlotinib | 4 | SD |
| 10 | Female/49 | No | Yes | Squamous cell carcinoma | IVb | Lung, liver, bone | PD-L1 + anlotinib | 4 | PD |
PD-1, programmed death 1; SD, stable response; PR, partial response; PD-L1, programmed death-ligand 1; PD, progressive disease.
Treatment response and survival analysis
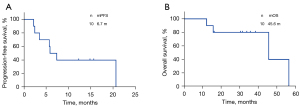
Among the ten patients analyzed, none achieved a CR, two achieved a PR, seven achieved SD and one achieved PD. The ORR and DCR were 20% and 90%, respectively. At the cutoff date, seven patients showed disease progression and four had died. Treatment with ICI and antiangiogenic drug combined therapy yielded a median PFS of 6.7 months [95% confidence interval (CI): 3.35–8.515, Figure 1A] and a median OS of 45.6 months (95% CI: 3.265–88.001, Figure 1B). Patients who received first- or second-line treatment had a longer median PFS and OS compared with those receiving third-line treatment or more; however, the P values were not significant (PFS: 10.32 vs. 6.68 months, P=0.67; OS: 56.47 vs. 45.63 months, P=0.12). No significant correlation was observed between PFS and sex (P=0.45), tumor histology (P=0.97), Masaoka stage (P=0.97), or surgery history (P=0.19). Figure 2 depicts the swimming plot for PFS.
Safety and tolerability
Table 3 presents the AEs that occurred during combination therapy. Treatment-related adverse events (TRAEs) occurred in 80% (8/10) of patients in this study and most were of grade 1–2. Proteinuria (40%) and an increase in glutamyl transpeptidase (GGT) (30%) were the most frequent AEs, followed by decreased appetite, fatigue, rash, hand-foot syndrome, and hypertension, which each occurred in 20% of patients (2/10). No grade 3 or higher AEs were observed, except for one patient receiving anti-PD-1 drugs paired with apatinib that developed a grade 3 rash. Apatinib medication was replaced with anlotinib in this patient.
Table 3
| AEs | Grade 1, n [%] | Grade 2, n [%] | Grade 3, n [%] |
|---|---|---|---|
| General disorders | |||
| Decreased appetite | 1 [10] | 1 [10] | |
| Fatigue | 2 [20] | ||
| Headache | 1 [10] | ||
| Dermatological toxicity | |||
| Rash | 1 [10] | 1 [10] | |
| Hand-foot syndrome | 1 [10] | 1 [10] | |
| Hematological toxicity | |||
| Thrombocytopenia | 1 [10] | ||
| Leukopenia | 1 [10] | ||
| Gastrointestinal toxicity | |||
| Diarrhea | 1 [10] | ||
| Endocrine toxicity | |||
| Hypothyroidism | 1 [10] | ||
| Renal toxicity | |||
| Proteinuria | 2 [20] | 2 [20] | |
| Hypertension | 1 [10] | 1 [10] | |
| Hepatotoxicity | |||
| GGT increased | 3 [30] | ||
| AST increased | 1 [10] | ||
| ALT increased | 1 [10] | ||
AEs, adverse events; PD-1, programmed death 1; GGT, glutamyl transpeptidase; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Association between TRAEs and treatment outcomes
The association between TRAEs and the efficacy of combination therapy was further explored. The median PFS of patients who developed TRAEs was longer than those without TRAEs (7.43 vs. 2.5 months, P=0.03). Patients without TRAEs had lower median OS rates compared with those with TRAEs, although this difference was not significant (15.8 vs. 45.6 months, P=0.32).
Discussion
In our study, immunotherapy combined with antiangiogenic therapy achieved an ORR of 20% and a median PFS of 6.7 months. These outcomes confirmed that ICIs plus antiangiogenic agents are an effective option for the treatment of advanced TETs, especially for early-line therapy during the process of clinical management.
At present, second-line and posterior-line treatment of patients with TETs lack standard drugs, and some studies have explored immune and antiangiogenic therapeutic strategies. Table 4 presents a summary of studies published in recent years on immunotherapy or antiangiogenic therapy for advanced TETs. According to previous studies, antiangiogenic monotherapy exhibited an ORR ranging from 6% to 40% and a median PFS ranging from 3.7 to 9.3 months in patients with TETs (6-8,12,13). A multi-center phase II clinical study by our group reported that apatinib yielded an ORR of 40%, a median PFS of 9.0 months, and an OS of 24 months among 25 patients with TETs (12). Our results were in accordance with prior studies on antiangiogenic therapy. With the development of immunotherapy, the efficacy of ICIs against advanced TETs has also been investigated. Pembrolizumab showed an ORR of 19.2% and PFS of 6.1 months in 26 patients with TC, compared with an ORR of 28.6% and PFS of 6.1 months in seven patients with Tm (4). Katsuya et al. reported that among fifteen TC patients in a PRIMER study, none achieved tumor shrinkage, with PFS and OS rates of 3.8 and 14.1 months, respectively (14). A multi-center retrospective study by our group found that combined immunotherapy was effective in advanced TC patients, especially those with high expression of PD-L1, with an ORR of 34.5% and median PFS of 8.0 months (15). The proportion of patients with stage III and IVA tumors in their study (54.5%) was larger than that in our study (20%). The combination of ICIs and antiangiogenic agents has proven potential in the treatment of several types of cancer (16). The CAVEATT study was the first study to report the use of immunotherapy in combination with antiangiogenic therapy in patients with TETs. This study reported an ORR of 34% in patients with advanced type B3 Tm and TC patients treated with axitinib plus avelumab, among which 41% (13/32) patients had received pretreatment with an anti-angiogenesis drug (17). Our study, which enrolled histologically-confirmed Tm or TC advanced patients, resulted in ORR and DCR rates of 20% and 90%, respectively, with median PFS and OS values of 6.7 and 45.6 months, respectively. In addition, patients treated earlier with ICIs combined with antiangiogenic targeting agents showed a higher median PFS. The results of our study were consistent with the results of the CAVEATT study; however, the CAVEATT study did not enroll Chinese patients, and thus efficacy data for the Chinese population are lacking. Because of the small sample size in our study, larger studies in the Chinese population are needed to determine whether these patients will benefit from combination therapies. Relevant, real-world research is ongoing. In patients previously treated with B3-Tm and TC, the phase II study PECATI (NCT04710628), which is continuing to test the clinical outcomes of lenvatinib coupled with pembrolizumab, is helping to support immunotherapy strategies for TETs (18).
Table 4
| Study regimen | Phase/clinical trial | Nation | N | Type of cancer | ORR | PFS/OS (months) |
|---|---|---|---|---|---|---|
| Pembrolizumab | II/NCT02607631 | Korea | 7 | Tm | 28.6 | 6.1/NR |
| 26 | TC | 19.2 | 6.1/14.5 | |||
| Pembrolizumab | II/NCT02364076 | USA | 40 | TC | 22.5 | 4.2/24.9 |
| Nivolumab | II/NCCH1505 | Japan | 15 | TC | 0 | 3.8/14.1 |
| Avelumab | I/NCT01772004 | USA | 8 | TETs | 57 | NA/NA |
| Sunitinib | II/NCT01621568 | USA | 23 | TC | 26 | 7.2/NR |
| 16 | Tm | 6 | 8.5/NR | |||
| Retrospective study | France | 28 | TETs | 22 | 3.7/15.4 | |
| Part of II/NCT01621568 | USA | 13 | TC | 8 | 5.0/16.0 | |
| Lenvatinib | II/UMIN000026777 | Japan | 42 | TC | 38 | 9.3/NR |
| Apatinib | II/ChiCTR-ONC-17013108 | China | 25 | TETs | 40 | 9.0/24.0 |
| Avelumab + axitinib | II/2017-004048-38 CAVEATT | Italy | 32 | TETs | 34 | 7.5/26.6 |
ORR, objective response rate; PFS, progression-free survival; OS, overall survival; Tm, thymomas; NR, not reached; TC, thymic carcinomas; TETs, thymic epithelial tumors; NA, not available.
The toxicity found in our study was in line with that reported in other solid tumor patients treated with ICIs plus antiangiogenic therapy (9,19). Some researchers have reported a high incidence (13% to 38%) of grade 3–4 AEs in patients with TETs (20). In our study, only one patient exhibited a grade 3 TRAE, with no grade 4 or higher TRAEs observed. A previous study on TETs patients indicated that immunotherapy increased the risk of autoimmune toxicity, with AEs such as myasthenia gravis (21). However, we did not observe autoimmune toxicity in our study. Proteinuria and hypertension were the main manifestations of toxicity related to antiangiogenic therapy in our study, both of which were grade 1 or grade 2. In summary, compared with immunotherapy or antiangiogenic monotherapy, combination therapy did not significantly increase related AEs and most AEs that did occur were reversible and manageable. Interestingly, we found that patients who developed TRAEs showed better median PFS than those who did not develop TRAEs. The association between the clinical effects and TRAEs warrants further investigation.
As a retrospective analysis, retrospective bias is unavoidable. The major limitation of our study is the small sample size. Hence, future studies with larger sample sizes are needed to explore the efficacy, toxicity, and biomarkers of this combination therapy regimen.
Conclusions
Patients with advanced TETs may receive some clinical benefit from ICIs combined with antiangiogenic targeting agents.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2192/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2192/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2192/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2192/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Carter BW, Benveniste MF, Madan R, et al. IASLC/ITMIG Staging System and Lymph Node Map for Thymic Epithelial Neoplasms. Radiographics 2017;37:758-76. [Crossref] [PubMed]
- Merveilleux du Vignaux C, Dansin E, Mhanna L, et al. Systemic Therapy in Advanced Thymic Epithelial Tumors: Insights from the RYTHMIC Prospective Cohort. J Thorac Oncol 2018;13:1762-70. [Crossref] [PubMed]
- Cho J, Kim HS, Ku BM, et al. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol 2019;37:2162-70. [Crossref] [PubMed]
- Szuchan C, Elson L, Alley E, et al. Checkpoint inhibitor-induced myocarditis and myasthenia gravis in a recurrent/metastatic thymic carcinoma patient: a case report. Eur Heart J Case Rep 2020;4:1-8. [Crossref] [PubMed]
- Rajan A, Kim C, Guha U, et al. OA18.02 Evaluation of a Modified Dosing Regimen (2-Weeks on/1-Week off) of Sunitinib as Part of a Phase II Trial in Thymic Carcinoma. J Thoracic Oncol 2017;12:S313-4. [Crossref]
- Sato J, Satouchi M, Itoh S, et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol 2020;21:843-50. [Crossref] [PubMed]
- Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015;16:177-86. [Crossref] [PubMed]
- Chu T, Zhong R, Zhong H, et al. Phase 1b Study of Sintilimab Plus Anlotinib as First-line Therapy in Patients With Advanced NSCLC. J Thorac Oncol 2021;16:643-52. [Crossref] [PubMed]
- Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020;21:571-80. [Crossref] [PubMed]
- Zhu AX, Finn RS, Ikeda M, et al. A phase Ib study of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC). J Clin Oncol 2020;38:abstr 4519.
- Song Z, Lou G, Wang Y, et al. Apatinib in patients with recurrent or metastatic thymic epithelial tumor: a single-arm, multicenter, open-label, phase II trial. BMC Med 2022;20:154. [Crossref] [PubMed]
- Remon J, Girard N, Mazieres J, et al. Sunitinib in patients with advanced thymic malignancies: Cohort from the French RYTHMIC network. Lung Cancer 2016;97:99-104. [Crossref] [PubMed]
- Katsuya Y, Horinouchi H, Seto T, et al. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer 2019;113:78-86. [Crossref] [PubMed]
- Wang W, Lin G, Hao Y, et al. Treatment outcomes and prognosis of immune checkpoint inhibitors therapy in patients with advanced thymic carcinoma: A multicentre retrospective study. Eur J Cancer 2022;174:21-30. [Crossref] [PubMed]
- Song Y, Fu Y, Xie Q, et al. Anti-angiogenic Agents in Combination With Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front Immunol 2020;11:1956. [Crossref] [PubMed]
- Conforti F, Zucali PA, Pala L, et al. Avelumab plus axitinib in unresectable or metastatic type B3 thymomas and thymic carcinomas (CAVEATT): a single-arm, multicentre, phase 2 trial. Lancet Oncol 2022;23:1287-96. [Crossref] [PubMed]
- Remon J, Girard N, Novello S, et al. PECATI: A Multicentric, Open-Label, Single-Arm Phase II Study to Evaluate the Efficacy and Safety of Pembrolizumab and Lenvatinib in Pretreated B3-Thymoma and Thymic Carcinoma Patients. Clin Lung Cancer 2022;23:e243-6. [Crossref] [PubMed]
- Taylor MH, Lee CH, Makker V, et al. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J Clin Oncol 2020;38:1154-63. [Crossref] [PubMed]
- Tateo V, Manuzzi L, De Giglio A, et al. Immunobiology of Thymic Epithelial Tumors: Implications for Immunotherapy with Immune Checkpoint Inhibitors. Int J Mol Sci 2020;21:9056. [Crossref] [PubMed]
- Zhao C, Rajan A. Immune checkpoint inhibitors for treatment of thymic epithelial tumors: how to maximize benefit and optimize risk? Mediastinum 2019;3:35. [Crossref] [PubMed]



