
An innovative risk index based on neutrophils and macrophages can effectively predict prognosis and immunotherapy response in patients with muscle-invasive bladder cancer
Highlight box
Key findings
• The innovative risk index based on tumor-infiltrating neutrophils and macrophages (M0, M2) can effectively predict prognosis and immunotherapy response in patients with muscle-invasive bladder cancer (MIBC).
What is known and what is new?
• MIBC is a highly heterogeneous malignancy, and its individualized treatment still faces serious difficulties and challenges. With extensive research on the tumor microenvironment, the important role of tumor-infiltrating immune cells (TIICs) in the progression and treatment of MIBC is now widely recognized.
• However, there is still a lack of prognostic models associated with TIICs for MIBC. Therefore, a novel prognostic risk index associated with TIICs for MIBC patients was constructed for the first time in this study.
What is the implication, and what should change now?
• The risk index is an independent prognostic factor for MIBC, which correlates with the effectiveness of immunotherapy and chemotherapy and can help to improve individualized treatment of MIBC patients. In addition to macrophages, this study suggests that neutrophils are an important potential therapeutic target for MIBC. However, the present model needs further validation in a prospective cohort study.
Introduction
Bladder cancer (BLCA) is the tenth most common cancer worldwide. According to the depth of tumor invasion, BLCA is divided into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). Compared with NMIBC, MIBC has invaded at least the muscularis tissue of the bladder and is more prone to lymph node and distant metastasis. Currently, the standard treatment for MIBC is still radical cystectomy (RC) with pelvic lymph node dissection (1). However, the treatment of MIBC faces serious difficulties and challenges. In fact, MIBC patients with the same TNM stage often have different prognostic levels, even with the same treatment regimen. In addition, although chemotherapy is an important treatment option, approximately 50% of MIBC patients are ineligible to cisplatin chemotherapy (2). Recent studies also have shown that the benefits of neoadjuvant chemotherapy are limited to only a subset of MIBC patients (2,3). Although the use of immune checkpoint blockade (ICB) therapy is a key emerging therapeutic strategy, their response rate in MIBC patients is only about 20% (4). According to statistics, the 5-year recurrence-free survival (RFS) and overall survival (OS) rates of MIBC patients after surgery were 68% and 60%, respectively, and these values were lower in patients with positive lymph nodes (5). Therefore, it is very important to develop an accurate prognostic biomarker in order to facilitate individualized treatment of MIBC patients (6).
With extensive research on the tumor microenvironment, researchers have realized the important role of tumor-infiltrating immune cells (TIICs) in tumor progression, metastasis, and treatment response (7). Indeed, the intratumoral infiltration of CD8+ T cells, Th1 CD4+ T cells, NK cells, and M1 macrophages (M1) is usually associated with a good prognosis, while Treg cells, Th2 CD4+ T cells, MDSCs, M2 macrophages (M2), and neutrophils (Neu) are often associated with poor prognosis (5). However, since the immunosuppressive microenvironment of tumors is the result of the combined action of these different types of immune cells, a single type of immune cells is not sufficient to accurately describe the impact of the immune microenvironment on the prognosis of cancer patients. The aim of this study was to construct a TIIC-based optimal prognostic risk index to guide further treatment of MIBC patients by means of bioinformatics.
The nomogram is a reliable and convenient statistical prediction tool that combines multiple variables to predict the endpoint of interest and has been widely used in a variety of solid tumors (8). Therefore, we combined the index with other clinical characteristics to establish a nomogram associated with the prognosis of MIBC patients. In conclusion, our study developed a novel immune-related index and confirmed that the index can effectively predict the prognosis and immunotherapy response of MIBC patients. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2255/rc).
Methods
Data acquisition and processing
The processing and normalization of research data were all achieved through the R software. First, we downloaded the clinical data and transcriptome sequencing data of 407 BLCA patients from the The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). According to the inclusion criteria, a total of 351 MIBC patients were included in this study for analysis. The following are the inclusion criteria in this study: (I) complete clinical information, such as age, gender, survival status, tumor node metastasis (TNM) stage, (II) T stage ≥T2, (III) histopathology belongs to urothelial carcinoma of bladder (UCB). According to the description of the “merged_sample_quality_annotations.tsv” file downloaded from the TCGA database, we excluded 7 non-UCB patients, (IV) the OS time ≥30 days. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
We downloaded two transcriptome matrices for TCGA-BCLA: fragments per kilobase of transcript per million mapped reads (FPKM) and Counts matrix. We used the “gencode.v38.annotation.gtf” file downloaded from the GENCODE database (https://www.gencodegenes.org/) to complete the gene annotation of the transcriptome matrices. Repeated genes were taken to average expression values, and low-expressed genes (no expression >40 samples) were removed. Then, we downloaded the “LM22.txt” file from the CIBERSORTx database (https://cibersortx.stanford.edu/), which contains reference marker genes for 22 immune cells. We used the FPKM matrix to evaluate the abundance of TIICs through the CIBERSORT algorithm, and used the Counts matrix to analyze differential genes between groups.
Construction of immune cell-related prognostic index
The CIBERSORT algorithm is an analytical tool from the Alizadeh Lab and Newman Lab to impute gene expression profiles and provide an estimation of the abundances of member cell types in a mixed cell population, using gene expression data. We used the “cibersort.R” script to obtain the abundance of 22 TIICs in 351 MIBC tissues. Then, the MIBC samples with CIBERSORT global deconvolution P<0.2 were further included to construct the risk index. We used the “ggplot2” package to visualize the distribution of different immune cells, and used “corrplot” to visualize the correlation between immune cells. Univariate Cox regression analysis was used to identify immune cells associated with OS. Only immune cells with P<0.2 in the univariate Cox regression analysis were included in the subsequent analysis. Subsequently, through least absolute shrinkage and selection operator (LASSO) Cox regression analysis and stepwise regression analysis, we obtained the best prognostic-related immune cells. Finally, we performed multivariate Cox regression analysis on the obtained immune cells and establish the prognostic risk index:
among them, “n” represents the number of immune cells, “βi” represents the regression coefficient of immune cells in the multivariate Cox regression analysis, and “Cellsi” are the abundance of immune cells.
Stratified analysis of the prognostic index
We calculated risk score for all included patients by the index and used their median as a cut-off value to classify MIBC patients into high- and low-risk groups. Kaplan-Meier (K-M) curves and log-rank tests were used for survival analysis between groups. In addition, all patients were divided into different subgroups based on different clinical characteristics. We analyzed the stratified performance of high- and low-risk patients in each subgroup by K-M curves. Moreover, we used the “timeROC” and “survival” packages to draw the receiver operating characteristic (ROC) curve of the risk score.
The prognostic index and immunotherapy response
IMvigor210 is an open-label, multicenter, single-arm phase II clinical study evaluating the efficacy and safety of PD-L1 antibody in locally advanced or metastatic urothelial carcinoma. We downloaded the IMvigor210 cohort-related data through the “IMvigor210CoreBiology” package in R software, and selected 148 patients whose histopathology was UCB as study subjects. Then, we used the same formula and method to evaluate the performance of the index. Additionally, we reveal the predictive value of the index for immunotherapy response by comparing the distribution of risk score across immunotherapy responses and immunophenotypes, respectively.
Establishment and evaluation of nomogram
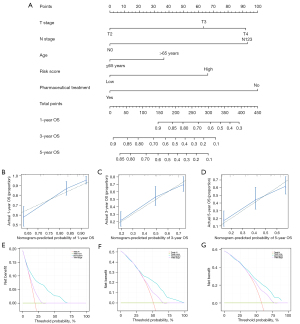
We identified clinical characteristics associated with OS by K-M curves. We then performed multivariate Cox regression analysis combining these clinical characteristics and the risk score, and finally identified independent prognostic factors. Then, through the “glmnet” package and the “rms” package, these independent prognostic factors were used to establish a nomogram. We used the consistency index (C-index), calibration curve and decision curve analysis (DCA) to verify the predictive accuracy and clinical benefit of the nomogram.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
We used the “limma” package to analyze differential genes between groups, and selected differential genes with adj.P.Val <0.05 & abs(logFC) >1 for GO and KEGG enrichment analysis. The GO and KEGG enrichment analysis were achieved by the “clusterProfiler” package. Moreover, these results were visualized through the “ggplot2” package, “ggrepel” package, “ggpubr” packages and “pheatmap” packages.
Statistical analysis
The correlation between TIICs was compared using Spearman’s rank correlation and t-test to verify. The CIBERSORT algorithm was used to calculate the cell abundance of 22 TIICs. Univariate Cox regression analysis, LASSO Cox regression analysis, and stepwise regression analysis were used to identify best prognostic-related TIICs. Multivariate Cox regression analysis was used to construct the risk index and nomograms. K-M curve and log-rank test were used to compare OS rates between groups. The ROC curve was used to evaluate the discriminatory power of the prognostic index. Finally, we evaluated the accuracy and clinical benefits of the nomogram through the C-index, calibration curve and DCA. All statistical calculations in this study were done in R-4.1.0 software.
Results
TIICs
We obtained the abundance of 22 TIICs in MIBC samples by the CIBERSORT algorithm. According to the standard of CIBERSORT global deconvolution P<0.2, 269 MIBC patients were selected to construct the risk index. In MIBC tissue, M0, M2 and CD8+ T cells were the most infiltrating immune cells (Figure 1A-1C). Resting mast cells was negatively correlated with activated mast cells (r=−0.67), activated NK cells was negatively correlated with resting NK cells (r=−0.46), M0 was negatively correlated with monocytes and CD8+ T cells (r=−0.52 and r=−0.46), CD8+ T cells was positively correlated with activated memory CD4+T cells (r=0.61), and plasma cells was positively correlated with naive B cells (r=0.52) (Figure 1D).
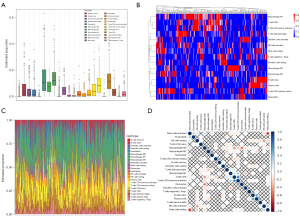
TIICs-based risk index
First, we identified TIICs associated with OS by univariate Cox regression analysis (P<0.2). They all include: plasma cells, CD8+ T cells, activated memory CD4+T cells, helper follicular T cells, M0, M2, activated mast cells, and Neu (Table 1). Through the LASSO regression analysis, we excluded CD8+ T cells and helper follicular T cells, which have multicollinearity problems (Figure 2A,2B). Finally, by stepwise regression analysis, M0, M2 and Neu were identified as the included variables for the best prognostic model. Then, the risk index using these TIICs as a variable was constructed by multivariate Cox regression analysis. The formula was as follows: Risk score = 1.63 × M0 + 2.33 × M2 + 11.6 × Neutrophils.
Table 1
| TIICs | HR (95% CI for HR) | P value |
|---|---|---|
| B.cells.naive | 0.46 (0.055–3.8) | 0.47 |
| B.cells.memory | 0.0032 (2.6e-07–39) | 0.23 |
| Plasma.cells | 0.045 (0.00073–2.7) | 0.14 |
| T.cells.CD8 | 0.086 (0.0099–0.74) | 0.025 |
| T.cells.CD4.naive | 580 (0.017–2e+07) | 0.23 |
| T.cells.CD4.memory.resting | 1.1 (0.11–11) | 0.93 |
| T.cells.CD4.memory.activated | 0.038 (0.0019–0.77) | 0.033 |
| T.cells.follicular.helper | 0.026 (0.00014–4.8) | 0.17 |
| T.cells.regulatory.Tregs. | 0.071 (0.00026–20) | 0.36 |
| T.cells.gamma.delta | 0.00022 (2.6e-11–1,800) | 0.3 |
| NK.cells.resting | 0.0076 (3.4e-06–17) | 0.21 |
| NK.cells.activated | 0.029 (5.6e-05–15) | 0.27 |
| Monocytes | 7 (0.00013–370,000) | 0.73 |
| Macrophages.M0 | 5.7 (1.6–20) | 0.0061 |
| Macrophages.M1 | 0.65 (0.021–20) | 0.81 |
| Macrophages.M2 | 11 (1.7–69) | 0.012 |
| Dendritic.cells.resting | 0.46 (0.023–9.2) | 0.61 |
| Dendritic.cells.activated | 0.58 (0.025–13) | 0.73 |
| Mast.cells.resting | 7.3 (0.22–240) | 0.27 |
| Mast.cells.activated | 210 (1.8–25,000) | 0.028 |
| Eosinophils | 8.3 (8.3e-07–8.3e+07) | 0.8 |
| Neutrophils | 74,000 (35–1.5e+08) | 0.004 |
TIICs, tumor-infiltrating immune cells; TCGA-MIBC, The Cancer Genome Atlas-muscle invasive bladder cancer; HR, hazard ratio; CI, confidence interval.
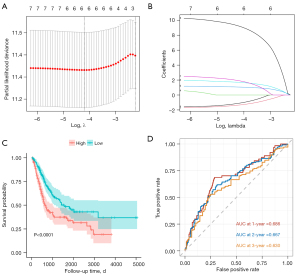
Stratification performance of the risk index
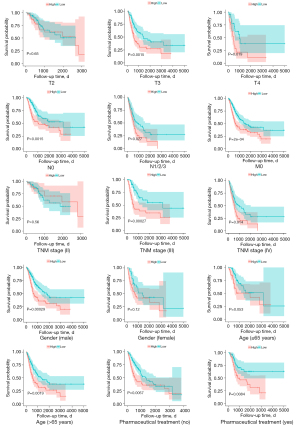
We used this formula to calculate the risk score for each included patient. Then, we divided the patients into high-risk group and low-risk group with the median of the risk score (0.573). K-M curves showed that patients in the low-risk group had a significantly higher OS rate compared with the high-risk group (P<0.0001) (Figure 2C). The area under curve (AUC) of the risk score was 0.686 at year 1, 0.667 at year 2, and 0.630 at year 3 (Figure 2D). Moreover, the risk score was well stratified in each of the following subgroups of patients [including T stage (T3), T stage (T4), N stage (N0), N stage (N1/2/3), M0, TNM (III), TNM (IV), gender (male), pharmaceutical treatment (yes)/(no), and age (>65 years)/(≤65 years)]. However, it performed poorly in the subgroups of T stage (T2), TNM stage (II), and gender (female) (Figure 3). Of note, we accidentally discovered that patients with distant metastases were all classified into the high-risk group. This made it impossible for us to do a stratification analysis of distant metastasis (M1).
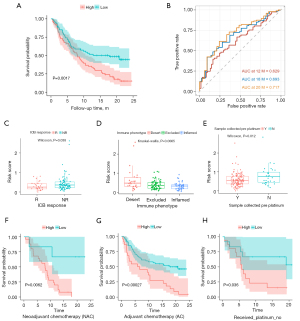
Correlation of risk index with immunotherapy and chemotherapy
In the IMvigor210 cohort, K-M survival curves also indicated that patients in the low-risk group had significantly higher OS rates than those in the high-risk group (P=0.0017, Figure 4A). The AUC of the risk score was 0.629 months 12, 0.693 months 18, and 0.717 months 20 (Figure 4B). Patients who did not respond to anti-PD-L1 immunotherapy had significantly higher risk score than those who responded (P=0.038, Figure 4C). In addition, the risk score was significantly different among the three tumor immunophenotypes (risk score: inflamed < excluded < desert, P=0.0065, Figure 4D). Moreover, boxplot showed that the risk score was significantly higher in the cisplatin-treated patient sample than in the untreated patients (P=0.012, Figure 4E). K-M survival curves showed that in the “neoadjuvant chemotherapy (NAC)” (P=0.0062, Figure 4F), “adjuvant chemotherapy (AC)” (P=0.00027, Figure 4G) and “Received_platinum_no” subgroups (P=0.036, Figure 4H), patients in the low-risk group had significantly higher OS rates than those in the high-risk group.
Nomogram based on the risk index
The K-M curve indicated that T stage, N stage, TNM stage, age, and pharmaceutical treatment were all clinical characteristics associated with OS (P<0.05, Figure S1). Considering the correlation between Tumor stage, N stage and TNM stage, we only kept the former for the next analysis. Because there were 28 patients whose pharmaceutical treatment was in the “not reported” status, we selected the data of the remaining 323 patients to construct a nomogram. Then, the results of multivariate Cox regression analysis of the risk score and prognosis-related clinical characteristics showed that the risk score, T stage, N stage, age, and pharmaceutical treatment were all independent prognostic factors (Figure S2), and they were used to establish a nomogram (Figure 5A). The C-index of the nomogram was 0.719 [95% confidence interval (CI): 0.696–0.742]. Calibration curves for 1, 3, and 5 years show good accuracy for the nomogram (Figure 5B-5D). Furthermore, DCA showed better clinical benefit of nomogram compared to the TNM staging system (Figure 5E-5G).
GO and KEGG function enrichment analysis
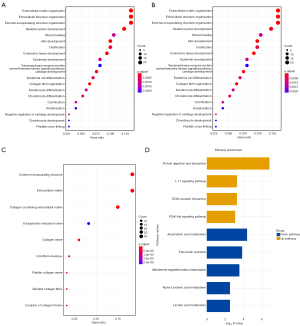
We obtained a total of 194 differential genes between the two groups in the TCGA-MIBC dataset, including 117 up-regulated genes and 77 down-regulated genes (Table S1). Volcano plot and heatmap were used to visualize the results (Figure S3). GO and pathway analysis showed that the up-regulated genes were mainly enriched in the processes of extracellular matrix organization, extracellular structure organization, external encapsulating structure organization, skeletal system development, wound healing and skin development (Figure 6A-6C). Additionally, the results of KEGG enrichment analysis showed that up-regulated genes were mainly enriched in protein digestion and absorption, IL-17 signaling pathway, extracellular matrix (ECM)-receptor interaction and PI3K-Akt signaling pathway, while down-regulated genes were mainly enriched in arachidonic acid metabolism, pancreatic secretion, aldosterone-regulated sodium reabsorption, alpha-linolenic acid metabolism and linoleic acid metabolism (Figure 6D).
Discussion
Tumor microenvironment (TME) usually includes many different immune cell subgroups with anti-tumor or tumor-promoting activities. A growing body of evidence shows that the interaction between TIICs and cancer cells affects the progression of cancer and the response to immunotherapy drugs. However, since the immunosuppressive microenvironment of tumors is the result of the combined action of different types of immune cells, a single type of immune cells is not sufficient to accurately describe the impact of the immune microenvironment on the prognosis of cancer patients. Therefore, this study is the first to construct a prognostic risk index based on TIICs for MIBC patients, aiming to improve the prediction level of prognosis and immunotherapy response, and promote individualized treatment of MIBC patients.
The risk index formula consists of three variables, including M0, M2, and Neu. Multivariate Cox regression analysis indicated that the index was an independent prognostic factor for MIBC patients. In fact, this may be because high infiltration levels of both macrophages (9-12) and neutrophils (13-16) are associated with tumor progression and poor prognosis. TME can promote the recruitment and polarization of macrophages in different ways. Although macrophages can be polarized into a series of phenotypes, the most common types are “M1” and “M2”. Both M1 and M2 are involved in the progression of tumors, but M1 plays a role in suppressing tumors through acute inflammation, while M2 promotes chronic inflammation and leads to immune suppression and tumor growth. At present, hundreds of cancer clinical trials targeting tumor-associated macrophages (TAMs) have been registered, including BLCA clinical trials (11). The main molecular mechanisms of these macrophage-targeted drugs include: inhibition of macrophage recruitment, depletion of TAMs, reprograming of TAMs, and activation of antitumor function of TAMs. Similar to TAMs, tumor-associated neutrophils (TANs) can also be polarized into anti-tumor (N1) or pro-tumor (N2) phenotypes. Interestingly, like TAMs, TANs often exhibit antitumor activity in the early stages of cancer, and gradually shift to pro-tumor activity during tumor progression (16-18). In addition, the latest research shows that neutrophils play a key role in the process of tumor metastasis (14). Fortunately, the efficacy of neutrophil-targeted drugs such as CXCL8 and CXCR1 blockers in solid tumors has entered clinical evaluation. Therefore, the risk index combines the predictive power of macrophages and neutrophils, which proves the reliability of the index.
In the stratification analysis, the risk score performed well in the subgroups of T stage (T3), T stage (T4), N stage (N0), N stage (N1/2/3), M0, TNM stage (III), TNM stage (IV), gender (male), pharmaceutical treatment (yes)/(no) and age (>65 years)/(≤65 years). However, they performed poorly in the subgroups of T2, TNM stage (II), and gender (female). The reasons for the poor performance of risk stratification in female patients may be related to the smaller sample size of female patients and more factors affecting the prognosis of female patients (19,20). As mentioned above, macrophages and neutrophils have anti-tumor activity in the early stages of tumors, and gradually transit to the tumor-promoting activities during tumor progression. This may account for the poor performance of the risk score in risk stratification in patients with earlier MIBC. The above results suggest that risk stratification of MIBC patients according to the risk score can help clinicians make decisions and improve patient outcomes. For example, good stratification performance in pharmaceutical treatment subgroup suggests that patients with lower risk score are more likely to benefit from the treatment.
With the in-depth study of TME, anti-tumor immunotherapy has been successfully developed and applied in patients with refractory or metastatic cancer. Among them, ICB therapy targeting CD8+ T cells has achieved significant clinical effects in some cancer patients (21). But in fact, the overall response rate of ICB therapy in MIBC patients is only about 20%. Studies have shown that the tumor immunosuppressive microenvironment can render ICB therapy ineffective by limiting the infiltration and activation of T effector cells (22,23). Therefore, we analyzed the association of the risk score with immunotherapy response through the IMvigor210 cohort. First, we confirmed that the risk score was associated with OS in these patients by K-M curves. Moreover, the ROC curve shows that the index has good discrimination as a predictor. Then, we found that the risk score were significantly different among the three immunophenotypes in MIBC (risk scores: inflamed < excluded < desert, P=0.0065). Finally, the risk score of patients who responded to PD-L1 antibody was significantly lower than that of patients who did not respond (P=0.038). Together, these results suggest that the risk score correlates with infiltration and activation of T effector cells and thus serves as a predictor of ICB treatment response.
Because the nomogram can predict a patient’s prognosis by combining multiple variables, it has shown higher predictive accuracy than the TNM staging system. In order to further improve the accuracy of prognosis prediction of MIBC patients, we combined TIICs-related index and clinical characteristics to establish a nomogram. The results of C-index and clinical decision curve show that the nomogram has good accuracy and calibration. In addition, the 1-, 3-, and 5-year DCA results showed that the nomogram we established had a higher clinical benefit compared with the TNM staging system.
Finally, the GO and KEGG enrichment analysis of the differential genes between high-risk group and low-risk group showed that up-regulated genes were enriched in protein digestion and absorption, IL-17 signaling pathway, ECM-receptor interaction, and PI3K-Akt signaling pathway. Down-regulated genes were enriched in the pathways of arachidonic acid metabolism, pancreatic secretion, aldosterone-regulated sodium reabsorption, alpha-linolenic acid metabolism, and linoleic acid metabolism. These pathways may be associated with tumor-promoting mechanisms of macrophages and neutrophils. It was found that IL-17 produced by γδT cells can promote neutrophil recruitment and polarization by regulating the release of granulocyto-colony-stimulating-factor (G-CSF) (24). Moreover, the production of IL-17 is induced by IL-1β secreted by TAMs (25). It is worth mentioning that in the cellular component (CC) analysis, the genes for collagen synthesis were the most expressed gene among the differential genes. In fact, recent studies found that collagen secreted by TAMs can activate the PI3K-Akt signaling pathway through integrin α2β1 to promote the progress of BLCA (11,26). In this study, down-regulated genes are mainly enriched in lipid metabolism pathways, which may lead to a high lipid state in the TME (27). Of note, the high lipid state in the TME can activate the immunosuppressive phenotype of macrophages and neutrophils, thereby promoting the progress of MIBC (28-30).
This study has some limitations. First, this study is a retrospective analysis of publicly available data, which leads to inevitable selection biases. Second, because our study is based on bioinformatic analysis, prospective clinical studies are needed to validate our findings. Third, we failed to incorporate other important prognostic factors in MIBC patients when establishing the nomogram. In future studies, we will try to incorporate more prognostic factors to improve the predictive effect of this nomogram.
Conclusions
In this study, we constructed a prognostic risk index based on TIICs for patients with MIBC. The index is an independent prognostic factor for MIBC patients and has good stratification performance. Furthermore, we demonstrated that the index is associated with immunotherapy response and has potential value in predicting immunotherapy efficacy. In conclusion, we developed a novel prognostic biomarker for MIBC patients that could facilitate individualized treatment of MIBC patients.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2255/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2255/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol 2021;79:82-104. [Crossref] [PubMed]
- Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin 2020;70:404-23. [Crossref] [PubMed]
- Ruiz de Porras V, Pardo JC, Etxaniz O, et al. Neoadjuvant therapy for muscle-invasive bladder cancer: Current clinical scenario, future perspectives, and unsolved questions. Crit Rev Oncol Hematol 2022;178:103795. [Crossref] [PubMed]
- Oh DY, Kwek SS, Raju SS, et al. Intratumoral CD4(+) T Cells Mediate Anti-tumor Cytotoxicity in Human Bladder Cancer. Cell 2020;181:1612-25.e13. [Crossref] [PubMed]
- Schneider AK, Chevalier MF, Derré L. The multifaceted immune regulation of bladder cancer. Nat Rev Urol 2019;16:613-30. [Crossref] [PubMed]
- Cathomas R, Lorch A, Bruins HM, et al. The 2021 Updated European Association of Urology Guidelines on Metastatic Urothelial Carcinoma. Eur Urol 2022;81:95-103. [Crossref] [PubMed]
- Tran L, Xiao JF, Agarwal N, et al. Advances in bladder cancer biology and therapy. Nat Rev Cancer 2021;21:104-21. [Crossref] [PubMed]
- Yang H, Zhang R, Zhang R, et al. Nomogram for distant metastasis-free survival in patients with locoregionally advanced nasopharyngeal carcinoma. Strahlenther Onkol 2022;198:828-37. [Crossref] [PubMed]
- Zhang X, Bai W, Hu L, et al. The pleiotropic mode and molecular mechanism of macrophages in promoting tumor progression and metastasis. Clin Transl Oncol 2023;25:91-104. [Crossref] [PubMed]
- Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 2018;17:887-904. [Crossref] [PubMed]
- Leblond MM, Zdimerova H, Desponds E, et al. Tumor-Associated Macrophages in Bladder Cancer: Biological Role, Impact on Therapeutic Response and Perspectives for Immunotherapy. Cancers (Basel) 2021;13:4712. [Crossref] [PubMed]
- Christofides A, Strauss L, Yeo A, et al. The complex role of tumor-infiltrating macrophages. Nat Immunol 2022;23:1148-56. [Crossref] [PubMed]
- Németh T, Sperandio M, Mócsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov 2020;19:253-75. [Crossref] [PubMed]
- Güç E, Pollard JW. Redefining macrophage and neutrophil biology in the metastatic cascade. Immunity 2021;54:885-902. [Crossref] [PubMed]
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016;16:431-46. [Crossref] [PubMed]
- Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol 2019;16:601-20. [Crossref] [PubMed]
- Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol 2019;19:255-65. [Crossref] [PubMed]
- Jaillon S, Ponzetta A, Di Mitri D, et al. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer 2020;20:485-503. [Crossref] [PubMed]
- Dobruch J, Daneshmand S, Fisch M, et al. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol 2016;69:300-10. [Crossref] [PubMed]
- Radkiewicz C, Edgren G, Johansson ALV, et al. Sex Differences in Urothelial Bladder Cancer Survival. Clin Genitourin Cancer 2020;18:26-34.e6. [Crossref] [PubMed]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Kersten K, Hu KH, Combes AJ, et al. Spatiotemporal co-dependency between macrophages and exhausted CD8(+) T cells in cancer. Cancer Cell 2022;40:624-38.e9. [Crossref] [PubMed]
- Zhang X, Du Y, Xiong W, et al. Combined single-cell RNA-seq and bulk RNA-seq to analyze the expression and role of TREM2 in bladder cancer. Med Oncol 2022;40:23. [Crossref] [PubMed]
- Coffelt SB, Kersten K, Doornebal CW, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015;522:345-8. [Crossref] [PubMed]
- Kersten K, Coffelt SB, Hoogstraat M, et al. Mammary tumor-derived CCL2 enhances pro-metastatic systemic inflammation through upregulation of IL1β in tumor-associated macrophages. Oncoimmunology 2017;6:e1334744. [Crossref] [PubMed]
- Qiu S, Deng L, Liao X, et al. Tumor-associated macrophages promote bladder tumor growth through PI3K/AKT signal induced by collagen. Cancer Sci 2019;110:2110-8. [Crossref] [PubMed]
- Masetti M, Carriero R, Portale F, et al. Lipid-loaded tumor-associated macrophages sustain tumor growth and invasiveness in prostate cancer. J Exp Med 2022;219:e20210564. [Crossref] [PubMed]
- Veglia F, Tyurin VA, Blasi M, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019;569:73-8. [Crossref] [PubMed]
- Al-Khami AA, Zheng L, Del Valle L, et al. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology 2017;6:e1344804. [Crossref] [PubMed]
- Di Conza G, Tsai CH, Gallart-Ayala H, et al. Tumor-induced reshuffling of lipid composition on the endoplasmic reticulum membrane sustains macrophage survival and pro-tumorigenic activity. Nat Immunol 2021;22:1403-15. [Crossref] [PubMed]


