
Surgical resection of primary tumors improves survival in patients with lung metastases: a population-based SEER analysis
Highlight box
Key findings
• The current study demonstrated that patients with lung metastases can benefit from SRPT.
What is known and what is new?
• SRPT resulted in better OS in several types of metastatic cancer.
• SRPT should be considered for lung metastatic patients.
What is the implication, and what should change now?
• In addition to radiotherapy and systemic chemotherapy, SRPT should also be seriously considered for lung metastatic patients. It is appreciated that the conclusion here will be validated and surgical inclusion criteria could be further clarified through well-designed, prospective, and randomized clinical trials.
Introduction
Distant metastases are the main cause of death in cancer patients, and are mostly found in the brain, liver, lungs, kidneys, and lymph nodes (1). The mechanism of distant metastasis is unclear. For cancer patients who exhibit no obvious symptoms, the tumors are difficult to be detected at earlier stage using present methods. Hence, for many patients, tumors were already in their advanced stages or had metastasized to other sites at the point of diagnosis, which further complicated the treatments. Many tumors are prone to distant metastases. For example, in osteosarcoma (2) and early-stage breast cancer (3), 13% and 20–30% of patients experienced distant metastases respectively. The lung is one of the most common metastatic sites for cancer metastasis, which accounts for 30–50% of all metastasis-related cases. Although distant lung metastases could occur in most types of cancer, they are most common in cancers involving melanoma, breast, colorectal, thyroid, head and neck and renal cell cancer (4). However, compared to other types of distant metastases, lung metastasized tumors have relatively lower growth rate and better overall survival (OS) (5). Therefore, treatment options for lung metastases could be different to that of metastases at other sites.
Although multiple treatment options, including locoregional and/or lung surgery, chemotherapy, immunotherapy and radiotherapy, could be used for lung metastatic cancer, the potential curative treatment approach would be comprehensive treatment for lung metastatic tumors. However, patients with lung metastasized tumors, which are already in advanced stages, are not recommended by the clinical guidelines to remove the primary tumors. Surgical resection of the primary tumor (SRPT) treated patients could have a 30–40% 5-year survival rate. Furthermore, previous studies revealed that SRPT resulted in better OS in several types of metastatic cancer, such as pancreatic cancer (6), gastroenteropancreatic neuroendocrine neoplasms (7), colorectal cancer (8), prostate cancer (9,10), and Ewing’s sarcoma (11). Nevertheless, none of these studies revealed a survival difference between lung metastases and other sites of metastases, as all these studies have relatively small sample size.
Given that the lungs are the predominant metastatic sites, it will be vital to understand how SRPT would affect the OS of patients with lung metastases. In the present study, we took advantage of the SEER database, which has a relatively large sample size, to assess whether SRPT should be considered for lung metastatic patients. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2459/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The data on cancer in the Surveillance, Epidemiology, and End Results (SEER) database is continually reported in every state of the United States and retrieved with no need for informed patient consent.
Patient cohort
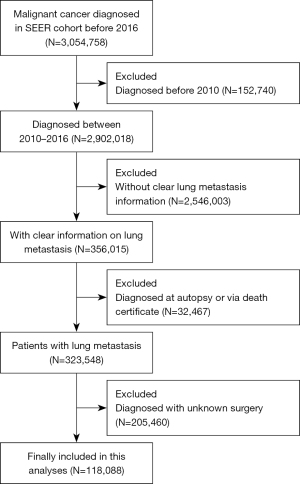
Eligible patients and their related information were obtained from the SEER database by employing a specific software (SEER*Stat, version 8.3.5, National Center Institute, USA). The SEER database recorded various clinical information including tumor characteristics, demographics, cancer incidence and prevalence, treatments and mortality. The SEER database has been widely used for clinical cancer studies. Patients diagnosed between 2010 and 2016 were included. The exclusion criteria were as following: (I) patients diagnosed before 2010 or after 2016, (II) diagnosed at autopsy or via death certificate, (III) patients with no clear lung metastasis information or surgery information. Detailed patient selection procedures are displayed in Figure 1.
Data collection
For each patient, the following clinical data were obtained: primary tumor type, age at diagnosis, gender, marital status, insurance status, income level, laterality, tumor grade, tumor stage, lymph node stage, metastasis sites (bone, brain, or liver), OS, and surgery status (yes or no). OS was used as the major endpoint outcome in the study. OS was defined as the duration from diagnosis to death due to any causes. Selected patients were classified into two subgroups (surgery and non-surgery). Additionally, 58 types of cancer identified from the SEER database were further divided into 13 cancer subtypes based on the primary tumor sites, including the oral cavity and pharynx, bones and joints, digestive, respiratory, soft tissue and skin, urinary, endocrine, male genital, female genital, blood, nervous, lymphatic, and the remaining systems.
Statistical analyses
Depending on different variables, differences in the clinical and demographic features between surgery and non-surgery groups were analyzed by the Fisher’s exact test, chi-squared test, or z-test. For each type of cancer, OS was evaluated by the Kaplan-Meier (K-M) estimator or a log-rank test. OS was further estimated by K-M curves. In addition, survival analyses were also performed for the 13 cancer subtypes. Median survival times and the associated 95% confidence intervals (CIs) were calculated by the K-M method. Multivariable survival analyses of OS were performed utilizing the Cox proportional hazards model. The loss to follow-up or follow-up interruption at the end of the observation was treated as a censoring event. Moreover, we applied a subgroup analysis to test the robustness of our results. For the above analyses, the following software was used: SPSS Statistics 22.0 software (IBM, NY, USA) for the chi-square test, z-test, K-M curves, log-rank test, and Cox regression analysis; GraphPad Prism 8.3 software (GraphPad, CA, USA) was used to generate the histograms; Comprehensive Meta-Analysis (CMA) Software 2.0 (Biostat, NJ, USA) was employed for the forest plotting. A two-tailed P value smaller than 0.05 was recognized as statistically significant.
Results
Patient and tumor characteristics
Detailed demographic and clinical parameters of the patients were summarized in Table 1; 118,088 selected patients with lung metastases were collected from the SEER database, of whom 18,688 (15.83%) had undergone surgery and 99,400 (84.17%) had not (Figure 1). The median age at diagnosis was 68.0 years; 77.85% and 94.18% of patients were white and insured, respectively. Selected patients in the non-surgery group were older than those in the surgery group (median age: 69.0 vs. 62.0 years, P<0.001). Younger age was also associated with improved OS from univariate and multivariable Cox proportional hazard regression analyses (Table 2). There was a larger proportion (41.37% vs. 28.98%) of patients with N0 stage cancer in the surgery group. Patients undergoing surgery were at lower T and N stage, but with higher grade. Univariate and multivariable Cox proportional hazard regression analyses showed that patients with lower N stage had a better OS (Table 2). Patients with cancer of the urinary (21.62%) and the female genital systems (12.92%) had higher ratio of SRPT. Moreover, the ratio of patients who underwent SRPT increased through the years from 2010 to 2016. This suggests that SRPT might be a mainstream treatment option for patients with lung metastasis in the future (Figure 2). Additionally, the survival rates in the surgery and non-surgery groups were 38.86% (n=7,263) and 16.19% (n=16,090), respectively.
Table 1
| Subject characteristics | Total, n (%) | Non-surgery, n (%) | Surgery, n (%) | χ2/Z | P value |
|---|---|---|---|---|---|
| All patients | 118,088 (100.00) | 99,400 (100.00) | 18,688(100.00) | ||
| Age, years | 71.33 | <0.001 | |||
| ≤18 | 979 (0.83) | 305 (0.31) | 674 (3.61) | ||
| 19–40 | 4,050 (3.43) | 2,281 (2.29) | 1,769 (9.47) | ||
| 41–64 | 42,972 (36.39) | 34,938 (35.15) | 8,024 (42.94) | ||
| ≥65 | 70,097 (59.35) | 61,876 (62.25) | 8,221 (43.99) | ||
| Sex | 86.82 | <0.001 | |||
| Male | 60,907 (51.58) | 51,713 (50.03) | 9,194 (49.20) | ||
| Female | 57,181 (48.42) | 47,687 (49.97) | 9,494 (50.80) | ||
| Race | 72.25 | <0.001 | |||
| White | 91,927 (77.85) | 77,172 (77.64) | 14,775 (79.06) | ||
| Black | 15,385 (13.03) | 13,198 (13.28) | 2,187 (11.70) | ||
| Others* | 10,482 (8.88) | 8,812 (8.87) | 1,670 (8.94) | ||
| Unknown | 274 (0.23) | 218 (0.22) | 56 (0.30) | ||
| Marital status | 1,295.19 | <0.001 | |||
| Married | 56,233 (47.62) | 46,897 (47.18) | 9,336 (49.96) | ||
| Unmarried | 56,431 (47.79) | 47,874 (48.16) | 8,557 (45.79) | ||
| Unknown | 5,424 (4.59) | 4,629 (4.66) | 795 (4.25) | ||
| Insurance | 66.20 | <0.001 | |||
| Insured | 111,218 (94.18) | 93,521 (94.09) | 17,697 (94.70) | ||
| Uninsured | 4,568 (3.87) | 3,841 (3.86) | 727 (3.89) | ||
| Unknown | 2,302 (1.95) | 2,038 (2.05) | 264 (1.41) | ||
| Income | 3.04 | 0.002 | |||
| <6,000 | 28,325 (23.99) | 24,090 (24.24) | 4,235 (22.66) | ||
| 6,000–7,000 | 35,641 (30.18) | 29,830 (30.01) | 5,811 (31.10) | ||
| 7,000–8,000 | 16,864 (14.28) | 14,103 (14.19) | 2,761 (14.77) | ||
| >8,000 | 37,255 (31.55) | 31,375 (31.56) | 5,880 (31.47) | ||
| Laterality | 1,951.55 | <0.001 | |||
| Right | 35,852 (30.36) | 31,169 (31.36) | 4,683 (25.06) | ||
| Left | 29,051 (24.60) | 24,577 (24.72) | 4,474 (23.94) | ||
| Bilateral | 3,687 (3.12) | 3,062 (3.08) | 625 (3.34) | ||
| Unknown | 49,498 (41.92) | 40,592 (40.84) | 8,906 (47.66) | ||
| Grade | 97.84 | <0.001 | |||
| I | 3,291 (2.79) | 2,571 (2.59) | 720 (3.85) | ||
| II | 16,654 (14.10) | 12,368 (12.44) | 4,286 (22.94) | ||
| III | 24,478 (20.73) | 19,297 (19.41) | 5,181 (27.73) | ||
| IV | 6,212 (5.26) | 2,885 (2.91) | 3,327 (17.80) | ||
| Unknown | 67,450 (57.12) | 62,277 (62.65) | 5,173 (27.68) | ||
| T stage | 68.69 | <0.001 | |||
| T1 | 11,166 (9.46) | 9,022 (9.08) | 2,144 (11.47) | ||
| T2 | 16,070 (13.61) | 12,732 (12.81) | 3,338 (17.86) | ||
| T3 | 28,352 (24.01) | 21,531 (21.66) | 6,821 (36.50) | ||
| T4 | 35,547 (30.10) | 31,058 (31.25) | 4,489 (24.02) | ||
| Unknown | 26,953 (22.82) | 25,057 (25.21) | 1,896 (10.15) | ||
| N stage | 63.24 | <0.001 | |||
| N0 | 36,542 (30.94) | 28,810 (28.98) | 7,732 (41.37) | ||
| N1 | 24,467 (20.72) | 19,316 (19.43) | 5,151 (27.56) | ||
| N2 | 25,395 (21.51) | 22,318 (22.45) | 3,077 (16.47) | ||
| N3 | 13,226 (11.20) | 12,429 (12.50) | 797 (4.26) | ||
| Unknown | 18,458 (15.63) | 16,527 (16.63) | 1,931 (10.33) | ||
| Bone MET | 3,186.79 | <0.001 | |||
| None | 81,588 (69.09) | 66,229 (66.63) | 15,359 (82.19) | ||
| Yes | 32,925 (27.88) | 29,940 (30.12) | 2,985 (15.97) | ||
| Unknown | 3,575 (3.03) | 3,231 (3.25) | 344 (1.84) | ||
| Brain MET | 2,184.96 | <0.001 | |||
| None | 99,823 (84.53) | 82,478 (81.98) | 17,345 (92.81) | ||
| Yes | 13,934 (17.80) | 13,005 (13.08) | 929 (4.97) | ||
| Unknown | 4,331 (3.67) | 3,917 (3.94) | 414 (2.22) | ||
| Liver MET | 1,168.42 | <0.001 | |||
| None | 76,782 (65.02) | 63,119 (63.50) | 13,663 (73.11) | ||
| Yes | 37,991 (32.17) | 33,269 (33.47) | 4,722 (25.27) | ||
| Unknown | 3,315 (2.81) | 3,012 (3.03) | 303 (1.62) | ||
| Cancer system | |||||
| Oral cavity and pharynx | 1,690 (1.43) | 1,440 (1.45) | 250 (1.34) | ||
| Digestive system | 31,829 (26.95) | 27,171 (27.34) | 4,658 (24.93) | ||
| Respiratory system | 47,679 (40.38) | 45,156 (45.43) | 1,523 (8.15) | ||
| Bones and joints | 626 (0.53) | 318 (0.32) | 308 (1.65) | ||
| Soft tissue including heart | 1,800 (1.52) | 1,154 (1.16) | 646 (3.46) | ||
| Skin excluding basal squamous | 2,777 (2.35) | 2,039 (2.05) | 738 (3.95) | ||
| Breast | 7,777 (6.59) | 6,090 (6.13) | 1,687 (9.03) | ||
| Female genital system | 6,478 (5.49) | 4,064 (4.09) | 2,414 (12.92) | ||
| Male genital system | 3,710 (3.14) | 2,330 (2.34) | 1,380 (7.38) | ||
| Urinary system | 10,652 (9.02) | 6,611 (6.65) | 4,041 (21.62) | ||
| Eye and orbit | 39 (0.03) | 20 (0.02) | 19 (0.10) | ||
| Brain and other nervous system | 63 (0.05) | 30 (0.03) | 33 (0.18) | ||
| Endocrine system | 1,768 (1.50) | 947 (0.95) | 821 (4.39) | ||
| Lymphoma | 571 (0.48) | 498 (0.50) | 73 (0.39) | ||
| Myeloma | 59 (0.05) | 50 (0.05) | 9 (0.05) | ||
| Leukemia | 111 (0.09) | 93 (0.09) | 18 (0.10) | ||
| Mesothelioma | 456 (0.39) | 298 (0.30) | 58 (0.31) | ||
| Kaposi sarcoma | 18 (0.02) | 16 (0.02) | 2 (0.01) | ||
| Miscellaneous | 85 (0.07) | 75 (0.08) | 10 (0.05) | ||
| Overall survival | 8,038.88 | <0.001 | |||
| Survival | 23,353 (19.78) | 16,090 (16.19) | 7,263 (38.86) | ||
| Death | 94,735 (80.22) | 83,310 (83.81) | 11,425 (61.14) |
*, American Indian/Alaska Native, Asian or Pacific Islander. MET, metastases.
Table 2
| Subject characteristics | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, years | |||||
| ≤18 | Reference | Reference | |||
| 19–40 | 1.822 (1.539–2.157) | <0.001 | 1.781 (1.500–2.116) | <0.001 | |
| 41–64 | 3.505 (3.005–4.088) | <0.001 | 2.877 (2.453–3.374) | <0.001 | |
| ≥65 | 4.826 (4.139–5.627) | <0.001 | 3.835 (3.269–4.499) | <0.001 | |
| Sex | |||||
| Male | Reference | Reference | |||
| Female | 0.935 (0.901–0.970) | <0.001 | 0.876 (0.843–0.909) | <0.001 | |
| Race | |||||
| White | Reference | Reference | |||
| Black | 1.133 (1.072–1.197) | <0.001 | 1.214 (1.148–1.284) | <0.001 | |
| Others* | 0.903 (0.845–0.966) | 0.003 | 0.910 (0.851–0.973) | 0.006 | |
| Unknown | NA | NA | |||
| Grade | |||||
| I | Reference | Reference | |||
| II | 1.364 (1.221–1.524) | <0.001 | 1.331 (1.190–1.488) | <0.001 | |
| III | 1.788 (1.603–1.994) | <0.001 | 2.004 (1.796–2.237) | <0.001 | |
| IV | 2.255 (2.017–2.521) | <0.001 | 2.962 (2.646–3.315) | <0.001 | |
| Unknown | NA | NA | |||
| T stage | |||||
| T1 | Reference | Reference | |||
| T2 | 1.256 (1.167–1.353) | <0.001 | 1.195 (1.109–1.288) | <0.001 | |
| T3 | 1.256 (1.176–1.343) | <0.001 | 1.104 (1.031–1.182) | 0.005 | |
| T4 | 1.818 (1.697–1.947) | <0.001 | 1.462 (1.361–1.570) | <0.001 | |
| Unknown | NA | NA | |||
| N stage | |||||
| N0 | Reference | Reference | |||
| N1 | 1.066 (1.019–1.115) | 0.006 | 1.087 (1.037–1.139) | <0.001 | |
| N2 | 1.356 (1.288–1.428) | <0.001 | 1.333 (1.261–1.409) | <0.001 | |
| N3 | 0.994 (0.903–1.095) | 0.904 | 1.139 (1.032–1.257) | 0.01 | |
| Unknown | NA | NA | |||
| Bone MET | |||||
| None | Reference | Reference | |||
| Yes | 1.494 (1.425–1.566) | <0.001 | 1.305 (1.242–1.371) | <0.001 | |
| Unknown | NA | NA | |||
| Brain MET | |||||
| None | Reference | Reference | |||
| Yes | 1.729 (1.602–1.867) | <0.001 | 1.826 (1.684–1.980) | <0.001 | |
| Unknown | NA | NA | |||
| Liver MET | |||||
| None | Reference | Reference | |||
| Yes | 1.679 (1.613–1.748) | <0.001 | 1.716 (1.643–1.792) | <0.001 | |
| Unknown | NA | NA | |||
| Radiotherapy | |||||
| No | Reference | Reference | |||
| Yes | 0.865 (0.827–0.905) | <0.001 | 0.853 (0.813–0.895) | <0.001 | |
| Unknown | NA | NA | |||
| Chemotherapy | |||||
| No | Reference | Reference | |||
| Yes | 0.587 (0.566–0.610) | <0.001 | 0.592 (0.569–0.616) | <0.001 | |
*, American Indian/Alaska Native, Asian or Pacific Islander. OR, odds ratio; CI, confidence interval; NA, not available; MET, metastases.
Survival analyses
Of the 118,088 patients, 94,735 (80.22%) were deceased by the end of follow-up. The median survival time was 19.0 months (95% CI: 18.45–19.55 months) and 4.0 months (95% CI: 3.94–4.06 months) for the surgery and non-surgery groups, respectively (P<0.001) (Table 3). After adjustment for parameters including age, gender, marital status, insurance status, income level, laterality, tumor grade, tumor stage, lymph node stage, metastasis sites (bone, brain, or liver), OS, and surgery status (yes or no), the multivariate Cox regression analysis indicated that recipients of SRPT were significantly related to a better OS [hazard ratio (HR) =0.49; 95% CI: 0.48–0.51, P<0.001] (Table 3).
Table 3
| Cancer system | Cancer site | Non-surgery (months), median (95% CI) | Surgery (months), median (95% CI) | Log Rank | P value | HR (95% CI) | P |
|---|---|---|---|---|---|---|---|
| – | All patients | 4.00 (3.94–4.06) | 19.00 (18.45–19.55) | 8792.07 | <0.001 | 0.49 (0.48–0.51) | <0.001 |
| Oral cavity and pharynx system | Lip | 1.00 (0.37–1.63) | 5.00 | 2.46 | 0.117 | 0.00 (0.00–0.01) | 0.013 |
| Tongue | 7.00 (5.28–8.72) | 11.00 (6.17–1.83) | 7.46 | 0.006 | 0.54 (0.37–0.80) | 0.002 | |
| Salivary gland | 8.00 (5.42–10.56) | 23.00 (16.93–29.07) | 27.19 | <0.001 | 0.38 (0.26–0.56) | <0.001 | |
| Floor of mouth | 5.00 (2.94–7.06) | 20.00 (4.90–35.11) | 8.12 | 0.004 | 0.28 (0.11–0.71) | 0.007 | |
| Gum and other mouth | 5.00 (3.47–6.53) | 8.00 (4.06–11.94) | 3.72 | 0.054 | 0.60 (0.36–1.00) | 0.051 | |
| Larynx | 6.00 (5.07–6.93) | 12.00 (8.86–15.14) | 11.61 | 0.001 | 0.55 (0.39–0.80) | 0.001 | |
| Nasopharynx | 13.00 (8.67–17.33) | 20.00 | 2.60 | 0.107 | 0.64 (0.25–1.64) | 0.411 | |
| Tonsil | 9.00 (7.50–10.51) | 17.00 (1.35–32.65) | 4.03 | 0.045 | 0.64 (0.36–1.13) | 0.122 | |
| Oropharynx | 5.00 (2.99–7.01) | 6.00 (0.00–12.20) | 1.21 | 0.271 | 0.65 (0.30–1.40) | 0.272 | |
| Hypopharynx | 6.00 (4.23–7.77) | 16.00 (3.60–28.40) | 4.59 | 0.032 | 0.50 (0.26–0.96) | 0.037 | |
| Other oral cavity and pharynx | 3.00 (1.24–4.76) | 12.00 (0.00–25.72) | 0.73 | 0.391 | 1.23 (0.35–4.29) | 0.742 | |
| Nose, nasal cavity and middle ear | 7.00 (0.78–13.22) | 14.00 (8.91–19.09) | 2.37 | 0.124 | 0.65 (0.31–1.34) | 0.241 | |
| Subtotal | 7.00 (6.42–7.58) | 16.00 (12.68–19.32) | 74.99 | <0.001 | 0.50 (0.43–0.58) | <0.001 | |
| Digestive system | Esophagus | 4.00 (3.72–4.29) | 10.00 (6.63–13.37) | 14.60 | <0.001 | 0.56 (0.40–0.79) | 0.001 |
| Stomach | 3.00 (2.72–3.28) | 6.00 (4.22–7.78) | 13.36 | <0.001 | 0.62 (0.50–0.77) | <0.001 | |
| Small intestine | 5.00 (3.36–6.64) | 12.00 (7.36–16.64) | 10.02 | 0.002 | 0.74 (0.55–1.01) | 0.059 | |
| Colon cancer | 4.00 (3.66–4.34) | 15.00 (13.94–16.06) | 739.75 | <0.001 | 0.58 (0.55–0.62) | <0.001 | |
| Rectum and rectosigmoid junction | 11.00 (10.27–11.73) | 26.00 (23.04–28.07) | 294.04 | <0.001 | 0.55 (0.50–0.60) | <0.001 | |
| Anus, anal canal and anorectum | 9.00 (6.80–11.20) | 10.00 (5.51–14.49) | 0.17 | 0.68 | 1.01 (0.69–1.48) | 0.970 | |
| Liver and intrahepatic bile duct | 2.00 (1.90–2.11) | 20.00 (11.33–28.67) | 178.43 | <0.001 | 0.29 (0.22–0.37) | <0.001 | |
| Gallbladder cancer | 3.00 (2.46–3.54) | 6.00 (3.67–8.33) | 4.89 | 0.027 | 0.74 (0.53–1.05) | 0.095 | |
| Other biliary | 2.00 (1.65–2.36) | 11.00 (4.56–17.44) | 14.46 | <0.001 | 0.58 (0.35–0.99) | 0.045 | |
| Pancreas | 2.00 (1.89–2.12) | 12.00 (8.45–15.55) | 79.77 | <0.001 | 0.41 (0.33–0.52) | <0.001 | |
| Retroperitoneum | 5.00 (2.52–7.48) | 18.00 (11.09–24.92) | 7.96 | 0.005 | 0.68 (0.44–1.05) | 0.078 | |
| Peritoneum, omentum and mesentery | 7.00 (3.69–10.31) | 29.00 (25.32–32.68) | 26.19 | <0.001 | 0.53 (0.36–0.79) | 0.002 | |
| Other digestive organs | 2.00 (1.77–2.23) | 6.00 (0.16–11.84) | 2.62 | 0.015 | 1.06 (0.50–2.23) | 0.876 | |
| Subtotal | 3.00 (2.92–3.08) | 17.00 (16.16–17.84) | 2653.93 | <0.001 | 0.47 (0.46–0.49) | <0.001 | |
| Respiratory system | Lung and bronchus | 4.00 (3.91–4.09) | 16.00 (14.19–17.81) | 752.60 | <0.001 | 0.42 (0.40–0.45) | <0.001 |
| Pleura | 0.00 | 3.00 | 0.14 | 0.702 | 0.08 | ||
| Trachea, mediastinum and other respiratory organs | 7.00 (4.35–9.66) | 17.00 (8.57–25.43) | 16.66 | <0.001 | 0.42 (0.25–0.71) | 0.001 | |
| Subtotal | 4.00 (3.91–4.09) | 16.00 (14.23–17.77) | 777.86 | <0.001 | 0.42 (0.40–0.45) | <0.001 | |
| Bones and joints system | Bones and joints | 10.00 (8.02–11.98) | 29.00 (23.00–35.00) | 59.78 | <0.001 | 0.62 (0.49–0.79) | <0.001 |
| Soft tissue and skin system | Melanoma of the skin | 5.00 (4.61–5.39) | 11.00 (9.60–12.40) | 72.16 | <0.001 | 0.70 (0.63–0.78) | <0.001 |
| Soft tissue including heart | 5.00 (4.35–5.65) | 16.00 (13.73–18.27) | 181.85 | <0.001 | 0.50 (0.44–0.57) | <0.001 | |
| Other non-epithlia skin | 6.00 (2.75–9.25) | 9.00 (6.31–11.69) | 0.98 | 0.323 | 0.62 (0.35–1.09) | 0.097 | |
| Mesothelioma | 4.00 (3.09–4.91) | 9.00 (5.99–12.01) | 9.21 | 0.002 | 0.72 (0.52–1.00) | 0.050 | |
| Eye and orbit | 4.00 (1.70–6.30) | 37.00 (4.26–69.74) | 6.32 | 0.012 | 0.80 (0.15–4.28) | 0.791 | |
| Kaposi sarcoma | 0.28 | 0.597 | 0.00 | 0.599 | |||
| Subtotal | 5.00 (4.68–5.32) | 13.00 (11.85–14.15) | 269.51 | <0.001 | 0.59 (0.54–0.63) | <0.001 | |
| Female genital system | Cervix uteri | 6.00 (5.34–6.66) | 12.00 (10.09–13.91) | 16.94 | <0.001 | 0.63 (0.48–0.82) | 0.001 |
| Breast cancer | 15.00 (14.11–15.89) | 29.00 (26.71–31.29) | 216.38 | <0.001 | 0.71 (0.66–0.76) | <0.001 | |
| Corpus and uterus, NOS | 3.00 (2.61–3.39) | 14.00 (12.60–15.40) | 341.18 | <0.001 | 0.50 (0.45–0.56) | <0.001 | |
| Ovary | 3.00 (2.53–3.47) | 30.00 (27.68–32.32) | 607.92 | <0.001 | 0.37 (0.32–0.43) | <0.001 | |
| Vagina | 5.00 (2.92–7.08) | 18.00 (2.81–33.19) | 2.15 | 0.143 | 0.68 (0.32–1.44) | 0.316 | |
| Vulva | 3.00 (1.90–4.11) | 6.00 (2.73–9.27) | 4.80 | 0.029 | 0.48 (0.30–0.75) | 0.001 | |
| Other female genital organs | 16.00 (7.05–24.95) | 47.00 (32.50–61.50) | 14.17 | <0.001 | 1.09 (0.69–1.74) | 0.708 | |
| Subtotal | 9.00 (8.53–9.47) | 24.00 (22.69–25.31) | 675.06 | <0.001 | 0.69 (0.66–0.73) | <0.001 | |
| Male genital system | Penis | 2.00 (0.21–3.79) | 7.00 (5.24–8.76) | 2.05 | 0.152 | 0.51 (0.20–1.34) | 0.170 |
| Testis | 8.00 (4.25–11.75) | 0.00 | 181.87 | <0.001 | 0.33 (0.24–0.47) | <0.001 | |
| Subtotal | 7.00 (3.31–10.69) | 204.66 | <0.001 | 0.37 (0.27–0.51) | <0.001 | ||
| Urinary system | Prostate | 12.00 (10.99–13.01) | 18.00 (14.19–21.81) | 11.36 | 0.001 | 0.84 (0.70–1.00) | 0.054 |
| Urinary | 2.00 (1.63–2.37) | 5.00 (4.46–5.54) | 54.33 | <0.001 | 0.83 (0.74–0.93) | 0.001 | |
| Kidney and renal pelvis | 4.00 (3.82–4.18) | 19.00 (17.47–20.54) | 1597.74 | <0.001 | 0.40 (0.37–0.43) | <0.001 | |
| Ureter | 4.00 (2.63–5.37) | 7.00 (5.18–8.82) | 4.27 | 0.039 | 0.90 (0.47–1.70) | 0.740 | |
| Other urinary organs | 2.00 (0.95–3.05) | 9.00 (7.13–10.88) | 2.69 | 0.101 | 0.76 (0.44–1.31) | 0.318 | |
| Subtotal | 5.00 (4.77–5.23) | 13.00 (12.30–13.70) | 837.30 | <0.001 | 0.69 (0.66–0.73) | <0.001 | |
| Nervous system | Cranial nerves other nervous system | 1.00 | 46.00 (0.00–106.01) | 1.10 | 0.294 | 0.00 (0.00–0.001) | 0.474 |
| Brain | 6.00 (0.00–15.79) | 7.00 (2.85–11.15) | 0.08 | 0.766 | 0.54 (0.20–1.48) | 0.230 | |
| Subtotal | 5.00 (0.00–11.09) | 10.00 (0.00–20.76) | 2.11 | 0.146 | 0.33 (0.15–0.76) | 0.009 | |
| Endocrine system | Other endocrine including thymus | 9.00 (6.27–11.74) | 48.00 (40.69–55.31) | 59.12 | <0.001 | 0.31 (0.23–0.42) | <0.001 |
| Thyroid | 2.00 (1.49–2.51) | 59.00 (41.39–70.61) | 412.80 | <0.001 | 0.28 (0.23–0.33) | <0.001 | |
| Subtotal | 4.00 (3.27–4.73) | 53.00 (43.22–63.78) | 409.71 | <0.001 | 0.26 (0.22–0.30) | <0.001 | |
| Lymphatic system | Non-Hodgkin lymphoma | 12.00 (8.52–15.48) | 32.00 (23.88–40.13) | 5.97 | 0.015 | 0.55 (0.35–0.87) | 0.010 |
| Hodgkin lymphoma | 1.43 | 0.232 | 1.72 (0.00–2.86) | 0.964 | |||
| Subtotal | 15.00 (11.50–18.50) | 32.00 (11.82–52.19) | 6.64 | 0.01 | 0.52 (0.33–0.82) | 0.004 | |
| Blood system | Leukemia | 6.00 (2.83–9.17) | 42.00 (20.61–63.39) | 15.75 | <0.001 | 0.31 (0.15–0.66) | 0.002 |
| Myeloma | 6.00 (1.61–10.39) | 7.00 (0.76–13.24) | 0.41 | 0.522 | 0.46 (0.12–1.78) | 0.262 | |
| Subtotal | 6.00 (3.43–8.57) | 27.00 (14.08–39.92) | 15.58 | <0.001 | 0.42 (0.23–0.77) | 0.005 | |
| Other system | Miscellaneous | 6.00 (1.89–10.11) | 23.00 (1.12–44.88) | 4.20 | 0.041 | 0.49 (0.20–1.17) | 0.107 |
Adjustment factors: adjusted for Ages at diagnosis, Sex, Marriage, Insurance, Race, Income, Grade, T stage, N stage, Radiation, Chemotherapy, Bone metastases, Brain metastases, Liver metastases. CI, confidence interval; HR, hazard ratio; NOS, not otherwise specified.
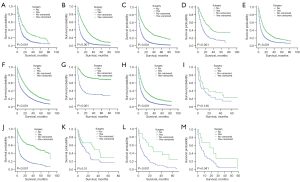
Of the 58 types of cancer, subjects who underwent SRPT had improved OS to varying degrees compared to those who did not. Moreover, the 58 types of cancer were divided into 13 subtypes and subjects with SRPT were associated with improved OS (Table 3). Among the patients whose primary tumors were in the oral cavity and pharynx system, median survival time was 16.00 months (95% CI: 12.68–19.32 months) and 7.00 months (95% CI: 6.42–7.58 months) for the surgery and non-surgery groups, respectively (P<0.001). Similarly, the median survival time of patients in the surgery group was significantly longer than that of those in the non-surgery group in cancer of the digestive system (17.00 months, 95% CI: 16.16–17.84 months vs. 3.00 months, 95% CI: 2.92–3.08 months; P<0.001), bones and joints (29.00 months, 95% CI: 23.00–35.00 months vs. 10.00 months, 95% CI: 8.02–11.98 months; P<0.001), soft tissue and skin (13.00 months, 95% CI: 11.85–14.15 months vs. 5.00 months, 95% CI: 4.68–5.32 months; P<0.001), female genital (24.00 months, 95% CI: 22.69–25.31 months vs. 9.00 months, 95% CI: 8.53–9.47 months; P<0.001), urinary (13.00 months, 95% CI: 12.30–13.70 months vs. 5.00 months, 95% CI: 4.77–5.23 months; P<0.001), endocrine (53.00 months, 95% CI: 43.22–63.78 months vs. 4.00 months, 95% CI: 3.27–4.73 months; P<0.001), lymphatic (32.00 months, 95% CI: 11.82–52.19 months vs. 15.00 months, 95% CI: 11.50–18.50 months; P=0.01), blood (27.00 months, 95% CI: 14.08–39.92 months vs. 6.00 months, 95% CI: 3.43–8.57 months; P<0.001), and the remaining miscellaneous systems (23.00 months, 95% CI: 1.12–44.88 months vs. 6.00 months, 95% CI: 1.89–10.11; P=0.041). However, a log-rank test revealed no difference of survival time between surgery and non-surgery groups in the nervous system (P=0.146). The K-M OS curves for the surgery and non-surgery groups were shown in Figure 3. At each time point, the survival time of patients in the surgery group was longer than that of those in the non-surgery group.
Patients who underwent SRPT exhibited a significant improvement in rate of survival, a finding observed across diverse synchronous metastases patterns (Table 4). In lung metastasis only, the median survival times of the surgery and non-surgery groups, were 15.00 months (95% CI: 14.56–15.44 months) and 5.00 months (95% CI: 4.90–5.11 months) (P<0.001), respectively. In lung metastasis combined with liver metastasis, the median survival times of the surgery and non-surgery groups, were 10.00 months (95% CI: 9.46–10.55 months) and 2.00 months (95% CI: 1.91–2.09 months) (P<0.001), respectively. In lung metastases combined with bone metastases, the median survival times were 10.00 months (95% CI: 9.26–10.74 months) and 4.00 months (95% CI: 3.86–4.14 months) for the surgery and non-surgery groups, respectively (P<0.001). Similarly, the median survival time of patients in the surgery group was significantly longer than that of patients in the non-surgery group in lung metastases combined with brain metastases [respectively, 8.00 months (95% CI: 6.98–9.02 months) and 3.00 months (95% CI: 2.82–3.18 months); P<0.001] (Table 4). Furthermore, most of the subjects had received primary tumour resection as the first treatment. No radiation and/or cancer-directed surgery accounted for 93.1%. Radiation prior to surgery accounted for 6.0%. Radiation after to surgery accounted for 0.7%. The sum of intraoperative radiation, intraoperative rad with other rad before/after and sequence unknown, but both were given accounted for 0.1% (Figure 4). Patients who underwent SRPT and chemotherapy or radiotherapy experienced a significant survival improvement. In chemotherapy, the median survival times were 24.00 months (95% CI: 23.29–24.71 months) and 9.00 months (95% CI: 8.87–9.13 months) for the surgery and non-surgery groups, respectively (P<0.001). In radiotherapy, the median survival times were 20.00 months (95% CI: 18.71–21.29 months) and 6.00 months (95% CI: 5.87–6.13 months) for the surgery and non-surgery groups, respectively (P<0.001). Patients who underwent chemotherapy or radiotherapy were also associated with improved OS from multivariable Cox proportional hazard regression analyses (Table 2).
Table 4
| Parameter | Non-surgery (months), median (95% CI) | Surgery (months), median (95% CI) | Log Rank | P value |
|---|---|---|---|---|
| Only lung metastasis | 5.00 (4.90–5.11) | 15.00 (14.56–15.44) | 3,834.12 | <0.001 |
| Lung + liver metastasis | 2.00 (1.91–2.09) | 10.00 (9.46–10.55) | 1,548.96 | <0.001 |
| Lung + bone metastasis | 4.00 (3.86–4.14) | 10.00 (9.26–10.74) | 442.61 | <0.001 |
| Lung + brain metastasis | 3.00 (2.82–3.18) | 8.00 (6.98–9.02) | 125.80 | <0.001 |
| Chemotherapy | ||||
| Yes | 9.00 (8.87–9.13) | 24.00 (23.29–24.71) | 7,849.03 | <0.001 |
| No | 1.00 (0.97–1.03) | 9.00 (8.37–9.63) | ||
| Radiotherapy | ||||
| Yes | 6.00 (5.87–6.13) | 20.00 (18.71–21.29) | 8,923.70 | <0.001 |
| No | 3.00 (2.94–3.06) | 18.00 (17.39–18.61) |
CI, confidence interval.
Multivariate Cox analyses in 13 subgroups displayed similar results. The HRs and 95% CIs for the surgery and non-surgery groups in each type of cancer were summarized in the forest plots (Figure 5). After adjustment for confounding factors, we found that SRPT improved OS among patients with cancer in the oral cavity and pharynx (HR =0.50; 95% CI: 0.43–0.58; P<0.001), digestive (HR =0.47; 95% CI: 0.46–0.49; P<0.001), bones and joints (HR =0.62; 95% CI: 0.49–0.79; P<0.001), female genital (HR =0.69; 95% CI: 0.66–0.73; P<0.001), soft tissue and skin (HR =0.59; 95% CI: 0.54–0.63; P<0.001), male genital (HR =0.37; 95% CI: 0.27–0.51; P<0.001), urinary (HR =0.69; 95% CI: 0.66–0.73; P<0.001), endocrine (HR =0.26; 95% CI: 0.22–0.30; P<0.001), nervous (HR =0.33; 95% CI: 0.15–0.76; P=0.009), lymphatic (HR =0.52; 95% CI: 0.33–0.82; P=0.004), and blood systems (HR =0.42; 95% CI: 0.23–0.77; P=0.005) (Table 3). Furthermore, we performed HR meta-analysis by using the random-effects model (Figure 5) and showed that SRPT was an independent prognostic factor corresponding to better OS (HR =0.34; 95% CI: 0.33–0.36; P<0.001).
Discussion
For decades, there is no effective therapy modality for patients with lung metastases. With the advancement of science and technology, new treatment methods, including systemic chemotherapy, radiotherapy, targeted therapy, immunotherapy and combination therapy, have emerged; however, their applicability and effectiveness are still limited. The curative treatment is still considered to be the complete removal of primary and metastatic tumors, and whether SRPT improves survival or not is still controversial. Thus, it is worth the in-depth investigation.
The results of the study demonstrated that there is a significant association between SRPT and better OS in patients with lung metastases based on the SEER dataset. The median survival time increased from 4.0 months in the non-surgery group to 19.0 months in the surgery group. Multivariate Cox regression analysis also validated that patients who received SRPT had a better OS. Thus, SRPT can be an alternative treatment strategy for cancer patients with lung metastases. The current study is believed to be the largest retrospective study that focused on evaluating the benefits of SRPT in patients with metastatic lung cancer.
In the subgroup analyses, we found that SRPT was beneficial for not only the general cancer patients but also the patients in each cancer subgroups. For primary tumors originated from the oral cavity and pharynx systems, the results suggested that SRPT could improve OS, which is consistent with previous reports. Harris et al. and Pan et al. suggested that compared to non-surgical patients, those who received SRPT showed a clear survival advantage with distant metastasis in laryngeal carcinoma (12,13). Tumors in the digestive system might cause bleeding, perforation, obstruction and malnutrition. SRPT can lower the risk of severe tumor-related complications and improve patients’ quality of life. Therefore, SRPT was recommended for patients with stage IV gastric cancer (14-16) and colorectal cancer (17,18). Additionally, Wang et al. also found that cancer patients with pancreas metastases who underwent SRPT had improved OS (19). Thus, the conclusion is also aligned with findings from previous studies.
The results of the study also showed that SRPT is associated with improved survival in surgery group with cancer in the bone and joint system. Likewise, several studies have found that SRPT improved OS of chondrosarcoma (20), osteosarcoma (21), and Ewing’s sarcoma in the bone system (11) with lung metastases. Melanoma is a type of skin cancer with increasing incidence and high malignancy (22). Melanoma patients with distant metastases usually have a worse prognosis regarding 5-year survival rates (<16%) and median survival time (<1 year) (23). The results of the study suggested that SRPT improved median survival time and OS for melanoma patients with lung metastases. A phase-2 clinical trial by Sosman et al. concluded that for certain selected patients, SRPT provided a promising treatment option that can improve OS of the patients (24). For breast cancer, the current study indicated that patients who received SRPT had improved OS. Some recent studies have also shown that SRPT in metastatic breast cancer was related to better OS (25,26). Moreover, SRPT has been shown to be effective in patients diagnosed with metastatic prostate (9), ovarian (27), and renal cell cancer (28). In addition, the current study also demonstrated that SRPT improved OS in other lung metastatic patients, which had not been reported previously.
The conclusions from the present study are consistent with that of the previous studies. We noticed that patients with metastatic lung cancer who received SRPT had improved OS. However, the underlying mechanisms are unclear. The possible mechanisms could be as following: (I) SRPT may reduce the number of blood circulating tumor cells which prevents micrometastases from becoming macrometastases (29,30) and eliminates the “seeding source” to blocking cancer progression (31); (II) SRPT may alleviate tumor burden to the body (32); (III) SRPT may associate with the recovery of the immune system by reversing systemic inflammation (33,34). In addition, SRPT can reduce severe tumor-related complications, and thus lead to better survival outcomes. The ratio of patients that had undergone SRPT was increasing from 2010 to 2016 except for 2015. This indicated that more and more lung metastasis patients tend to choose SRPT as a treatment option. With the advancement systemic therapies, surgical techniques and imaging techniques, distant metastases at earlier stages would be more capable of being detected. Further, neoadjuvant therapy provides another opportunity for clinicians to perform SRPT on these patients (35). In addition, the development of imaging techniques can help the clinicians to visualize the tumor borders and blood supply more clearly and thus help in developing surgical protocols that could remove tumors more safely and thoroughly.
Although the current study is supported by detailed analysis, it has limitations in several ways. Firstly, considering that the study design was based solely on the SEER database, it could not avoid the potential inherent subject selection bias. Secondly, the SEER database lacks information for factors such as disease burden, surgical margin status, preoperative status, smoking status, alcohol intake and other detailed factors (e.g., the presence of comorbidities and complications). How these factors would affect OS is unknown, which may influence the surgical decisions. It was not known for the reasons why the patients underwent SRPT. Thirdly, some factors were not described in detail in the SEER database. For example, types of surgery, whether palliative or radical surgery, open operation or minimally invasive surgery, were unknown. Chemicals and their doses used for chemotherapy or the radiation strategies used were also unknown. These unrecorded factors might affect the outcomes of survival analyses. Fourthly, apart from lung metastases, it is possible to combine data for metastases at other sites, which might have an impact on OS of patients. Finally, the SEER database does not provide computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), B-ultrasound and other imaging data, which has a certain impact on the evaluation of whether to perform surgical treatment and the prognosis. Despite the aforementioned limitations, this population-based study included a large number of lung metastasis patients and showed significant correlations with rigorous analyses, and thus should be very convincing and conclusive.
Conclusions
The results of the study showed that SRPT is effective for patients with lung metastases. However, at present, the criteria for suitability of undergoing surgery are unknown. Therefore, it is appreciated that the conclusion here will be validated and surgical inclusion criteria could be further clarified through well-designed, prospective, and randomized clinical trials. Finally, in addition to radiotherapy and systemic chemotherapy, SRPT should also be seriously considered for lung metastatic patients.
Acknowledgments
The authors acknowledge all the staff for their great efforts in the SEER program.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2459/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2459/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The data on cancer in the SEER database is continually reported in every state of the United States and retrieved with no need for informed patient consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559-64. [Crossref] [PubMed]
- Wang W, Yang J, Wang Y, et al. Survival and prognostic factors in Chinese patients with osteosarcoma: 13-year experience in 365 patients treated at a single institution. Pathol Res Pract 2017;213:119-25. [Crossref] [PubMed]
- Chen W, Hoffmann AD, Liu H, et al. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. NPJ Precis Oncol 2018;2:4. [Crossref] [PubMed]
- Seo JB, Im JG, Goo JM, et al. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics 2001;21:403-17. [Crossref] [PubMed]
- Prasanna T, Karapetis CS, Roder D, et al. The survival outcome of patients with metastatic colorectal cancer based on the site of metastases and the impact of molecular markers and site of primary cancer on metastatic pattern. Acta Oncol 2018;57:1438-44. [Crossref] [PubMed]
- Feng T, Lv W, Yuan M, et al. Surgical resection of the primary tumor leads to prolonged survival in metastatic pancreatic neuroendocrine carcinoma. World J Surg Oncol 2019;17:54. [Crossref] [PubMed]
- Tsilimigras DI, Hyer JM, Paredes AZ, et al. Resection of Primary Gastrointestinal Neuroendocrine Tumor Among Patients with Non-Resected Metastases Is Associated with Improved Survival: A SEER-Medicare Analysis. J Gastrointest Surg 2021;25:2368-76. [Crossref] [PubMed]
- Peng P, Luan Y, Sun P, et al. Prognostic Factors in Stage IV Colorectal Cancer Patients With Resection of Liver and/or Pulmonary Metastases: A Population-Based Cohort Study. Front Oncol 2022;12:850937. [Crossref] [PubMed]
- Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol 2014;65:1058-66. [Crossref] [PubMed]
- Jin K, Qiu S, Jin H, et al. Survival Outcomes for Metastatic Prostate Cancer Patients Treated With Radical Prostatectomy or Radiation Therapy: A SEER-based Study. Clin Genitourin Cancer 2020;18:e705-22. [Crossref] [PubMed]
- Ren Y, Zhang Z, Shang L, et al. Surgical Resection of Primary Ewing's Sarcoma of Bone Improves Overall Survival in Patients Presenting with Metastasis. Med Sci Monit 2019;25:1254-62. [Crossref] [PubMed]
- Harris BN, Bhuskute AA, Rao S, et al. Primary surgery for advanced-stage laryngeal cancer: A stage and subsite-specific survival analysis. Head Neck 2016;38:1380-6. [Crossref] [PubMed]
- Pan Y, Hong Y, Liang Z, et al. Survival analysis of distant metastasis of laryngeal carcinoma: analysis based on SEER database. Eur Arch Otorhinolaryngol 2019;276:193-201. [Crossref] [PubMed]
- Thrumurthy SG, Chaudry MA, Chau I, et al. Does surgery have a role in managing incurable gastric cancer? Nat Rev Clin Oncol 2015;12:676-82. [Crossref] [PubMed]
- Li Z, Ren H, Wang T, et al. Resection of the Primary Tumor Improves the Survival of Patients With Stage IV Gastric Neuroendocrine Carcinoma. Front Oncol 2022;12:930491. [Crossref] [PubMed]
- Xu J, Lu D, Zhang L, et al. Palliative resection or radiation of primary tumor prolonged survival for metastatic esophageal cancer. Cancer Med 2019;8:7253-64. [Crossref] [PubMed]
- Yi C, Li J, Tang F, et al. Is Primary Tumor Excision and Specific Metastases Sites Resection Associated With Improved Survival in Stage IV Colorectal Cancer? Results From SEER Database Analysis. Am Surg 2020;86:499-507. [Crossref] [PubMed]
- Yao YC, Chen JQ, Yin L, et al. Primary tumor resection with or without metastasectomy for left- and right-sided stage IV colorectal cancer: an instrumental variable analysis. BMC Gastroenterol 2022;22:114. [Crossref] [PubMed]
- Wang L, Yang L, Chen L, et al. Do Patients Diagnosed with Metastatic Pancreatic Cancer Benefit from Primary Tumor Surgery? A Propensity-Adjusted, Population-Based Surveillance, Epidemiology and End Results (SEER) Analysis. Med Sci Monit 2019;25:8230-41. [Crossref] [PubMed]
- Song K, Song J, Chen F, et al. Does Resection of the Primary Tumor Improve Survival in Patients With Metastatic Chondrosarcoma? Clin Orthop Relat Res 2019;477:573-83. [Crossref] [PubMed]
- Huang X, Zhao J, Bai J, et al. Risk and clinicopathological features of osteosarcoma metastasis to the lung: A population-based study. J Bone Oncol 2019;16:100230. [Crossref] [PubMed]
- Abdel-Rahman O. Clinical correlates and prognostic value of different metastatic sites in patients with malignant melanoma of the skin: a SEER database analysis. J Dermatolog Treat 2018;29:176-81. [Crossref] [PubMed]
- Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008;26:527-34. [Crossref] [PubMed]
- Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430. Cancer 2011;117:4740-06. [Crossref] [PubMed]
- Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol 2015;16:1380-8. [Crossref] [PubMed]
- Wang Z, Chen B, Chen J, et al. A Novel Nomogram Model to Identify Candidates and Predict the Possibility of Benefit From Primary Tumor Resection Among Female Patients With Metastatic Infiltrating Duct Carcinoma of the Breast: A Large Cohort Study. Front Oncol 2022;12:798016. [Crossref] [PubMed]
- Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002;20:1248-59. [Crossref] [PubMed]
- Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 2001;358:966-70. [Crossref] [PubMed]
- Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res 2006;12:6403-9. [Crossref] [PubMed]
- Wong NS, Kahn HJ, Zhang L, et al. Prognostic significance of circulating tumour cells enumerated after filtration enrichment in early and metastatic breast cancer patients. Breast Cancer Res Treat 2006;99:63-9. [Crossref] [PubMed]
- Pockaj BA, Wasif N, Dueck AC, et al. Metastasectomy and surgical resection of the primary tumor in patients with stage IV breast cancer: time for a second look? Ann Surg Oncol 2010;17:2419-26. [Crossref] [PubMed]
- Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery 2002;132:620-6; discussion 626-7. [Crossref] [PubMed]
- DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev 2010;29:309-16. [Crossref] [PubMed]
- Turner N, Tran B, Tran PV, et al. Primary Tumor Resection in Patients With Metastatic Colorectal Cancer Is Associated With Reversal of Systemic Inflammation and Improved Survival. Clin Colorectal Cancer 2015;14:185-91. [Crossref] [PubMed]
- Barnes CA, Aldakkak M, Clarke CN, et al. Value of Pretreatment 18F-fluorodeoxyglucose Positron Emission Tomography in Patients With Localized Pancreatic Cancer Treated With Neoadjuvant Therapy. Front Oncol 2020;10:500. [Crossref] [PubMed]





