
Clinical outcomes of hepatocellular carcinoma patients after hepatectomy treated with TACE in combination with sorafenib: a propensity score matched analysis
Highlight box
Key findings
• Transarterial chemoembolization (TACE) + sorafenib did not significantly enhance OS in individuals who had had hepatectomy compared to TACE alone; however, time to target tumor progression was noticeably improved.
What is known and what is new?
• A prolonged duration of sorafenib treatment leads to clinical benefits.
• TACE plus sorafenib or systemic drug-TACE sequential therapy may be a viable therapeutic choice for patients who experience recurrence following hepatectomy.
What is the implication, and what should change now?
• Patients who have recurrence after hepatectomy may benefit from sequential TACE therapy with sorafenib or systemic drug-TACE therapy. Individuals with poor TACE results who have relapsed after hepatectomy may be candidates for co-administration of sorafenib.
Introduction
Hepatic resection is regarded as the first-line therapy for patients with hepatocellular carcinoma (HCC); notably, recurrence after resection remains a significant problem with many series reporting rates of 60–70% at 5 years (1-4). Bilobar tumor lesions, extrahepatic metastases, and portal vein invasion have been associated with a high propensity for tumor recurrence after resections (5-9). Patients with recurrent HCC are at increased risk of secondary surgery due to the smaller size of the remaining liver after initial resection and the significantly reduced reserve and compensatory capacity of the liver. Furthermore, taking into consideration issues like quantity and location of recurring lesions unsuitable for surgery, most patients will get systemic or intra-arterial therapies (10,11).
Patients with significant unresectable or multifocal HCC without a main or lobar branch portal vein thrombus who are not candidates for local ablation might consider transarterial chemoembolization (TACE) (12-14). TACE is based on inducing tumor ischemia by cutting off the blood supply of the tumor. As a side effect of this treatment, hypoxia inducible factors are expressed by tumor after TACE (15). Angiopoietin-2 and vascular endothelial growth factor are upregulated by elevated levels of hypoxia-inducible factor 1, which worsens the prognosis for patients by promoting intrahepatic metastasis, extrahepatic metastasis, and vascular invasion (16).
A multikinase inhibitor known as sorafenib targets platelet-derived growth factor receptor-beta as well as vascular endothelial growth factor receptors 1/2/3 (17). Sorafenib has been shown to increase survival when compared to optimal supportive treatment alone. It has been proposed that giving sorafenib might help target the overexpression of TACE-induced angiogenic factors and therefore enhance the results of TACE therapy (18). The pertinent question is whether adding systemic therapy improves outcomes relative to locoregional therapy alone for individuals with liver-isolated HCC who are qualified for liver-directed nonsurgical interventions. Even though sorafenib has been the only systemic medication licensed for the treatment of HCC for 10 years, information on recurrent HCC following hepatic resection is scant.
Therefore, this study aimed to evaluate whether a combination of TACE and sorafenib can improve the outcomes in recurrent unresectable HCC patients after initial resection. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2784/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical Colleges (No. NCC-010299) and informed consent was obtained from all the patients.
Patient population
All patients with recurrent unresectable HCC who received TACE as first-line therapy at National Clinical Research Center for Cancer/Cancer Hospital from 2010 to 2020 were identified from an electronic database for a retrospective analysis. After a multidisciplinary tumor board debate, patients were chosen for TACE, and treatment was started after obtaining the written informed permission.
The inclusion criteria of this study were as follows: (I) patients with their first recurrence after liver resection; (II) age >18 years; (III) treated with TACE alone or TACE combined with sorafenib; (IV) sorafenib treatment for at least 4 weeks; (V) with Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1; (VI) with Child-Pugh classification of A or B. The exclusion criteria of this study are as follows: (I) patients with other malignant tumor diseases; (II) patients with serious medical comorbidities; (III) patients who received other targeting agents or immunotherapy; (IV) the period between the administration of sorafenib and the initial TACE procedure was more than a month; (V) patients who had undergone other treatments before this study; (VI) unavailability of medical records.
TACE protocol
Regularly using the Seldinger procedure, the 5 F catheter was inserted after puncturing the femoral artery. In order to identify the tumor target vessel during angiography, the catheter was positioned near the celiac trunk artery’s entrance. Into the tumor-supplying artery, super selective intubation was carried out. Oxaliplatin (50–100 mg), pirarubicin (10–40 mg), and lipiodol made up the therapy protocol (2–20 mL). The medication was chosen at the doctor’s discretion, and the amount of lipiodol was determined by the size of the tumor. Following the operation, hepatoprotective hydration therapy was administered.
Sorafenib management
All patients received comprehensive information about the sorafenib therapy, including details about its effectiveness, potential side effects, and cost. Sorafenib treatment (daily dosage, 400 mg b.i.d.) was started 2–30 days after the first TACE and maintained until intolerance, refusal, or tumor progression occurred. Based on the existence of toxicity, the dose of sorafenib was reduced. If grade 3 or 4 adverse effects (AEs) occurred, a dosage change (400 mg once a day) was undertaken until the AEs were eased or eliminated. If grade 3 or 4 adverse events persisted following dosage modification, sorafenib therapy would be discontinued until the AEs were relieved or eliminated.
Follow-up
Following treatment, the standard of care clinical and radiological follow-up was set at one month and then every three months. All patients had triphasic computed tomography (CT) examinations at the initial follow-up, and subsequent follow-ups were done with either CT or magnetic resonance imaging (MRI). Retreatment was only undertaken on demand, following a multidisciplinary tumor board discussion of all available alternatives, based on the extent of remaining or recurring viable tumors and the clinical circumstances of the patients.
Data analysis
The following factors were gathered: baseline age, sex, liver function, and tumor extension; baseline demographic and clinical data; laboratory tests; periprocedural complications; target and overall tumor response; radiological tumor progression; and survival.
The main outcome in this trial was overall survival (OS), which was defined as the period from when the HCC recurrence was identified to when the patient died for any reason. The secondary outcome was time to target tumor progression (TTTP). Treatment responses were classified as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) using the modified Response Evaluation Criteria in Solid Tumors (mRECIST). Two radiologists discussed and validated the results of contrast-enhanced CT or MRI scans.
Statistical analysis
PSM of TACE + S to TACE patients was performed with a caliper width of 0.1 and 1:1 nearest-neighbor matching. After matching, the balance of covariate distribution across groups was examined using the standardized mean difference (SMD). Data were investigated using descriptive statistics (mean and standard deviation) then compared using chi-square or Fisher exact tests for categorical data and Student’s t-test for continuous variables. The log-rank test was used to compare the estimated Kaplan-Meier and OS curves. Using Cox proportional hazards models, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Regardless of eligibility or treatment length, all patients who received at least one dosage of the prescribed therapy were included in the safety analysis. A P value of 0.05 was used as the threshold for statistical significance in the statistical analysis, which was performed using specialized software (SAS, Cary; and Stata, Stata Corp).
Results
Patient demographic and clinical characteristics
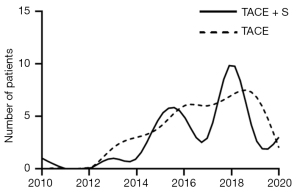
There were 381 recurrent patients with unresectable HCC who satisfied the inclusion criteria in total, and 234 of them were disqualified based on the exclusion criteria (Figure 1). Figure 2 displays the number of patients recruited over time. The median follow-up time was 33.1 months.
The study population’s demographic and baseline parameters are displayed in Table 1. Prior to propensity matching, 32 patients received TACE combined with sorafenib compared to 115 with TACE alone. Based on the 32 patients who accepted sorafenib, 32 patients with TACE alone were matched for the analysis using the closest available neighbor approach (1:1). There were no appreciable distinctions between the two groups’ baseline characteristics in the PSM cohort.
Table 1
| Variables | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| TACE alone | TACE + sorafenib | SMDΔ | TACE alone | TACE + sorafenib | SMDΔ | ||
| N | 115 | 32 | 32 | 32 | |||
| Age (years) | 58.27±11.88 | 58.09±8.79 | −0.020 | 56.56±13.10 | 58.09±8.79 | 0.174 | |
| Gender | |||||||
| Female | 10 (8.7) | 5 (15.6) | 0.191 | 6 (18.8) | 5 (15.6) | −0.086 | |
| Male | 105 (91.3) | 27 (84.4) | −0.191 | 26 (81.2) | 27 (84.4) | 0.086 | |
| Viral hepatitis | |||||||
| HBV | 99 (86.1) | 24 (75.0) | −0.256 | 23 (71.9) | 24 (75.0) | 0.072 | |
| HCV | 5 (4.3) | 0 (0.0) | −0.240 | 0 (0.0) | 0 (0.0) | ||
| No infections | 11 (9.6) | 8 (25.0) | 0.356 | 9 (28.1) | 8 (25.0) | −0.072 | |
| Child-Pugh score | |||||||
| A | 112 (97.4) | 31 (96.9) | −0.030 | 32 (100.0) | 31 (96.9) | −0.180 | |
| B | 3 (2.6) | 1 (3.1) | 0.030 | 0 (0.0) | 1 (3.1) | 0.180 | |
| ECOG PS | |||||||
| 0 | 114 (99.1) | 32 (100.0) | 0.106 | 32 (100.0) | 32 (100.0) | 0.000 | |
| 1 | 1 (0.9) | 0 (0.0) | −0.106 | 0 (0.0) | 0 (0.0) | 0.000 | |
| AFP | |||||||
| <400 | 96 (83.5) | 26 (81.2) | −0.057 | 25 (78.1) | 26 (81.2) | 0.080 | |
| ≥400 | 19 (16.5) | 6 (18.8) | 0.057 | 7 (21.9) | 6 (18.8) | −0.080 | |
| Number of nodules | |||||||
| Multiple-diffuse | 69 (60.0) | 24 (75.0) | 0.346 | 22 (68.8) | 24 (75.0) | 0.144 | |
| Single | 46 (40.0) | 8 (25.0) | −0.346 | 10 (31.2) | 8 (25.0) | −0.144 | |
| Vascular invasion | |||||||
| No | 101 (87.8) | 24 (75.0) | −0.296 | 25 (78.1) | 24 (75.0) | −0.072 | |
| Yes | 14 (12.2) | 8 (25.0) | 0.296 | 7 (21.9) | 8 (25.0) | 0.072 | |
| Extrahepatic metastasis | |||||||
| No | 102 (88.7) | 25 (78.1) | −0.256 | 26 (81.2) | 25 (78.1) | −0.076 | |
| Yes | 13 (11.3) | 7 (21.9) | 0.256 | 6 (18.8) | 7 (21.9) | 0.076 | |
Values are listed as number (percentage) or mean ± standard deviation unless otherwise stated. TACE, transarterial chemoembolization; SMD, standardized mean difference; HBV, hepatitis B virus; HCV, hepatitis C virus; ECOG PS, Eastern Cooperative Oncology Group Performance Status; AFP, alpha-fetoprotein.
Tumor progression and survival in the matched population
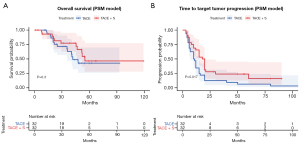
For propensity-matched patients, the combination treatment group and the monotherapy group underwent a Kaplan-Meier analysis to determine OS and TTTP. At the cut-off date of September 1, 2022, 25 OS events were observed. The median OS for the TACE + sorafenib group was 48.5 months (95% CI: 39.01–58.05), compared to 41.0 months (95% CI: 34.17–53.83) for the TACE monotherapy group. As a result, TACE + sorafenib did not substantially improve survival compared to TACE alone (P=0.300) (Figure 3). The estimated median TTTP was 10.5 months (95% CI: 9.24–25.88) after TACE and 19.0 months (95% CI: 15.79–31.83) after TACE + sorafenib (P=0.017). Significantly longer TTTP was observed in the TACE plus sorafenib arm than in the TACE alone arm.
Prognostic factors for OS in the PSM cohort
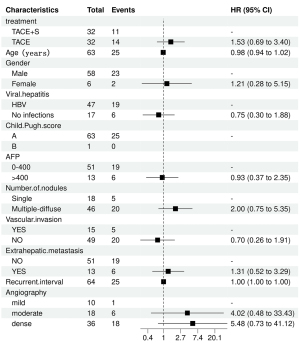
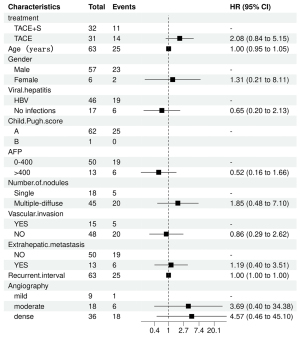
Table 2 provides the results of the univariate and multivariate studies of the OS-influencing factors. The PSM cohort’s univariate log-rank test analysis revealed that OS was related to the number of nodules and the degree of arterial enhancement. Multivariate Cox regression analysis revealed that TACE in combination with sorafenib did not substantially improve survival compared to TACE alone (HR =2.08; 95% CI: 0.84–5.15; P=0.11). Figures 4,5 show forest plots of the results of the one-way and multi-way Cox regression analyses respectively.
Table 2
| Characteristic | Univariable | Multivariable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Event N | HR | 95% CI | P value | N | Event N | HR | 95% CI | P value | ||
| Treatment | |||||||||||
| TACE + sorafenib | 32 | 11 | – | – | 32 | 11 | – | – | |||
| TACE | 32 | 14 | 1.53 | 0.69, 3.40 | 0.30 | 31 | 14 | 2.08 | 0.84, 5.15 | 0.11 | |
| Age (years) | 63 | 25 | 0.98 | 0.94, 1.02 | 0.25 | 63 | 25 | 1.00 | 0.95, 1.05 | 0.93 | |
| Gender | |||||||||||
| Male | 58 | 23 | – | – | 57 | 23 | – | – | |||
| Female | 6 | 2 | 1.21 | 0.28, 5.15 | 0.80 | 6 | 2 | 1.31 | 0.21, 8.11 | 0.77 | |
| Viral hepatitis | |||||||||||
| No infections | 17 | 6 | – | – | 17 | 6 | – | – | |||
| HBV | 47 | 19 | 1.33 | 0.53, 3.34 | 0.54 | 46 | 19 | 1.55 | 0.47, 5.09 | 0.47 | |
| Child-Pugh score | |||||||||||
| A | 63 | 25 | – | – | 62 | 25 | – | – | |||
| B | 1 | 0 | 1 | 0 | |||||||
| AFP | |||||||||||
| <400 | 51 | 19 | – | – | 50 | 19 | – | – | |||
| ≥400 | 13 | 6 | 0.93 | 0.37, 2.35 | 0.87 | 13 | 6 | 0.52 | 0.16, 1.66 | 0.27 | |
| Vascular invasion | |||||||||||
| No | 49 | 20 | – | – | 48 | 20 | – | – | |||
| Yes | 15 | 5 | 1.42 | 0.52, 3.85 | 0.49 | 15 | 5 | 1.16 | 0.38, 3.50 | 0.80 | |
| Number of nodules | |||||||||||
| Single | 18 | 5 | – | – | 18 | 5 | – | – | |||
| Multiple-diffuse | 46 | 20 | 2.00 | 0.75, 5.35 | 0.04 | 45 | 20 | 1.85 | 0.48, 7.10 | 0.27 | |
| Extrahepatic metastasis | |||||||||||
| No | 51 | 19 | – | – | 50 | 19 | – | – | |||
| Yes | 13 | 6 | 1.31 | 0.52, 3.29 | 0.56 | 13 | 6 | 1.19 | 0.40, 3.51 | 0.76 | |
| Recurrent interval | 64 | 25 | 1.00 | 1.00, 1.00 | 0.28 | 63 | 25 | 1.00 | 1.00, 1.00 | 0.59 | |
| Angiography | |||||||||||
| Mild | 10 | 1 | – | – | 9 | 1 | – | – | |||
| Moderate | 18 | 6 | 4.02 | 0.48, 33.4 | 0.10 | 18 | 6 | 3.69 | 0.40, 34.4 | 0.20 | |
| Dense | 36 | 18 | 5.48 | 0.73, 41.1 | 0.03 | 36 | 18 | 4.57 | 0.46, 45.1 | 0.11 | |
HR, hazard ratio; CI, confidence interval; TACE, transarterial chemoembolization; HBV, hepatic B virus; AFP, alpha-fetoprotein.
Safety outcomes
AEs that resulted from the treatment are displayed in Table 3. Fatigue (71.9%) and abdominal pain (65.6%) were the two AEs that occurred the most frequently in the TACE alone group. Additionally, one patient (3.1%) experienced abdominal pain in grade 3/4. Hand-foot skin reaction (81.3%), abdominal pain (71.9%), and fatigue (62.5%) were the three most common AEs in the TACE plus sorafenib group. The most frequent grade 3/4 AEs were abdominal pain (3.1%), vomiting (3.1%), and fever (3.1%). In neither group was there a mortality connected to the treatment.
Table 3
| Adverse events | TACE + sorafenib (n=32) | TACE alone (n=32) | |||
|---|---|---|---|---|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | ||
| Diarrhea | 18 | 0 | 12 | 0 | |
| Abdominal pain | 22 | 1 | 20 | 1 | |
| Ascites | 2 | 0 | 3 | 0 | |
| Vomiting | 10 | 1 | 7 | 0 | |
| Fatigue | 20 | 0 | 23 | 0 | |
| Fever | 13 | 1 | 11 | 0 | |
| Headache | 5 | 0 | 6 | 0 | |
| Upper respiratory infection | 2 | 0 | 2 | 0 | |
| PLT decreased | 3 | 0 | 4 | 0 | |
| WBC decreased | 2 | 0 | 3 | 0 | |
| Anaemia | 2 | 0 | 3 | 0 | |
| Hand-foot skin reaction | 26 | 0 | 0 | 0 | |
| Hypertension | 5 | 0 | 0 | 0 | |
| Hair loss | 1 | 0 | 0 | 0 | |
| Gastrointestinal hemorrhage | 2 | 0 | 1 | 0 | |
| Epistaxis | 1 | 0 | 0 | 0 | |
TACE, transarterial chemoembolization; PLT, platelet; WBC, white blood cell.
Discussion
To maximize therapeutic results, recurrent HCC typically necessitates intensive therapy. TACE is the most often utilized therapy for recurrent HCC after resection. With the publication of data revealing that the molecularly targeted drug sorafenib increases survival compared to optimal supportive treatment alone, there has been a renaissance of excitement and interest for systemic therapy of HCC (19). Real-world data are still missing, nevertheless, addressing the administration of concurrent multi-modality therapy and how this can affect resource usage and long-term effects. The results from the current study show that the concurrent treatment of sorafenib may postpone, but not avoid, tumor development following TACE.
We choose a time frame for our investigation between 2010 and 2020. This is connected to the fact that sorafenib was introduced to our institution in 2010, with an inevitable early learning curve caused by the absence of international expertise. Sorafenib is a multikinase inhibitor that, among other things, inhibits the vascular endothelial growth factor receptor. Prospective randomized studies have confirmed that in HCC in the intermediate stage, TACE plus sorafenib may be an option for treatment (19,20). The SHARP trial provided the foundation for the United States’ approval of sorafenib for the systemic treatment of advanced HCC and positioned sorafenib monotherapy as a novel reference standard. In our study, recurring individuals with unresectable HCC received S-TACE (sorafenib-TACE) combination treatment with a minimal benefit over TACE monotherapy. The conclusions of a significant amount of earlier research in the TACTICS experiment are supported by these results (21). Findings from the TACTICS trial showed that TACE with sorafenib did not significantly improve OS compared to TACE alone in patients with advanced HCC. Nonetheless, when TACE and sorafenib were combined, as opposed to sorafenib alone, there was a significant improvement in tumor morphology, as evidenced by better PRs and a decrease in the incidence of PD. Given the potential of sorafenib to inhibit TACE-induced VEGF angiogenesis released by acute hypoxia, it seems that sorafenib might have stabilised disease progression after TACE session, leading to a prolonged TTTP. We tentatively put forward that effective subsequent therapy was a major factor contributing to the lack of a significant OS benefit in this trial.
The current analysis showed that the main risk factors for poor survival included the number (≥2) and the degree of arterial enhancement of the recurrent HCCs. The Child-Pugh class, vascular invasion, and tumor size have all been found to be independent predictors of outcomes in earlier studies. These findings are consistent with those findings (22,23). Interestingly, alpha-fetoprotein (AFP) was not a significant prognostic factor for survival according to the univariate analysis. This outcome might be explained by the study’s limited number of cases.
The most frequent AEs reported with sorafenib usage were hand-foot skin reaction, diarrhea, alopecia, and tiredness, according to evidence from recent trials (24-28). Similar safety findings were established in the current investigation, which showed that hand-foot skin reaction was the most frequently reported drug-emergent AE, followed by abdominal pain and fatigue. In our study, the majority of these AEs were grade 1/2 and well tolerated, which seldom led to medication cessation.
This study’s primary drawback stems from its retrospective methodology. Since some patients’ medical records could not be located, follow-up was not uniform, and some information on locoregional and systemic treatments delivered by doctors in other institutions could have been overlooked, several individuals were removed from the study. The time frame used for the study is another drawback. Thirdly, it was unfortunate that this study only involved a limited number of patients. To validate the findings of this study, more patients must participate in external validation studies.
Despite these drawbacks, the approach of this study adds fresh information to this comparison while correlating some of the data provided by sizable prospective randomized trials (19,20,29). TACE + sorafenib failed to significantly increase OS when compared to TACE. However, considering the much enhanced lasting local tumor control that was noted, patients may benefit from using TACE with sorafenib. Future research with a larger sample size will be needed to determine whether TACE plus sorafenib is beneficial for patients with a greater liver reserve.
Conclusions
In conclusion, there was no statistically significant increase in OS with TACE plus sorafenib. Because of the considerable increase in TTTP, TACE plus sorafenib or systemic drug-TACE sequential therapy may be a viable therapeutic choice for patients who experience recurrence following hepatectomy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2784/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2784/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2784/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical Colleges (No. NCC-010299) and informed consent was obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bruix J, Sherman MAmerican Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999;229:216-22. [Crossref] [PubMed]
- Lee EC, Kim SH, Park H, et al. Survival analysis after liver resection for hepatocellular carcinoma: A consecutive cohort of 1002 patients. J Gastroenterol Hepatol 2017;32:1055-63. [Crossref] [PubMed]
- Roayaie S, Bassi D, Tarchi P, et al. Second hepatic resection for recurrent hepatocellular cancer: a Western experience. J Hepatol 2011;55:346-50. [Crossref] [PubMed]
- Lee KF, Chong CCN, Fong AKW, et al. Pattern of disease recurrence and its implications for postoperative surveillance after curative hepatectomy for hepatocellular carcinoma: experience from a single center. Hepatobiliary Surg Nutr 2018;7:320-30. [Crossref] [PubMed]
- Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015;261:947-55. [Crossref] [PubMed]
- Lee SY, Konstantinidis IT, Eaton AA, et al. Predicting recurrence patterns after resection of hepatocellular cancer. HPB (Oxford) 2014;16:943-53. [Crossref] [PubMed]
- Cha C, Fong Y, Jarnagin WR, et al. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003;197:753-8. [Crossref] [PubMed]
- Ferris JV, Baron RL, Marsh JW Jr, et al. Recurrent hepatocellular carcinoma after liver transplantation: spectrum of CT findings and recurrence patterns. Radiology 1996;198:233-8. [Crossref] [PubMed]
- Kudo M, Arizumi T, Ueshima K, et al. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: Proposal of Modified Bolondi's Subclassification (Kinki Criteria). Dig Dis 2015;33:751-8. [Crossref] [PubMed]
- Kudo M, Han KH, Ye SL, et al. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020;9:245-60. [Crossref] [PubMed]
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [Crossref] [PubMed]
- Pelletier G, Ducreux M, Gay F, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol 1998;29:129-34. [Crossref] [PubMed]
- Bargellini I, Sacco R, Bozzi E, et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol 2012;81:1173-8. [Crossref] [PubMed]
- Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol 2008;103:914-21. [Crossref] [PubMed]
- Wilson GK, Brimacombe CL, Rowe IA, et al. A dual role for hypoxia inducible factor-1α in the hepatitis C virus lifecycle and hepatoma migration. J Hepatol 2012;56:803-9. [Crossref] [PubMed]
- Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 2006;66:11851-8. [Crossref] [PubMed]
- Erhardt A, Kolligs F, Dollinger M, et al. TACE plus sorafenib for the treatment of hepatocellular carcinoma: results of the multicenter, phase II SOCRATES trial. Cancer Chemother Pharmacol 2014;74:947-54. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Kudo M, Ueshima K, Ikeda M, et al. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer 2022;11:354-67. [Crossref] [PubMed]
- Zheng J, Shao G, Luo J. Analysis of survival factors in patients with intermediate-advanced hepatocellular carcinoma treated with transcatheter arterial chemoembolization combined with sorafenib. Clin Transl Oncol 2014;16:1012-7. [Crossref] [PubMed]
- Ohki T, Sato K, Yamagami M, et al. Erratum to: Efficacy of Transcatheter Arterial Chemoembolization Followed by Sorafenib for Intermediate/Advanced Hepatocellular Carcinoma in Patients in Japan: A Retrospective Analysis. Clin Drug Investig 2016;36:93-6. [Crossref] [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9. [Crossref] [PubMed]
- Veronese ML, Mosenkis A, Flaherty KT, et al. Mechanisms of hypertension associated with BAY 43-9006. J Clin Oncol 2006;24:1363-9. [Crossref] [PubMed]
- Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol 2008;19:1955-61. [Crossref] [PubMed]
- Autier J, Escudier B, Wechsler J, et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol 2008;144:886-92. [Crossref] [PubMed]
- Lencioni R, Kudo M, Ye SL, et al. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int J Clin Pract 2012;66:675-83. [Crossref] [PubMed]
- Raoul JL, Bruix J, Greten TF, et al. Relationship between baseline hepatic status and outcome, and effect of sorafenib on liver function: SHARP trial subanalyses. J Hepatol 2012;56:1080-8. [Crossref] [PubMed]



