
Moving molecular subtypes to the clinic in gastric cancer
Introduction
Adenocarcinomas of the stomach and gastroesophageal junction account for a high proportion of worldwide cancer-related mortality. Some progress has been made with the approval of HER2 and VEGFR2 targeted therapies, though the overall prognosis remains poor in metastatic disease with median overall survivals of 12−16 months (1,2). With the well-established understanding that cancer is a disease process owing to the unfettered growth of cells that stems from acquired somatic or germline DNA alterations, multiple investigators have queried the genome to gain insights into novel therapies.
Recently, gastric cancer has been put under the genetoscope and distinct molecularly-defined subtypes have emerged (3-7). Prior to molecular classification gastric cancer has largely been characterized by anatomic location and histologic subtype according to Lauren and WHO classification schema (8,9). While histopathologic analyses have observed differing features and prognoses between diffuse and intestinal type gastric adenocarcinomas, genomic data adds detailed information about underlying operative mutational processes and highlights recurrent changes with therapeutic implications.
In the article by Li et al., published in the April 1, 2016 issue of Cancer Research, the authors conduct an in depth analysis of genomic level data pooled from five large sequencing studies to establish a study set of 544 annotated gastric cancer specimens (10). Using aggregated genomic data, the authors sought to increase sensitivity to identify additional recurrently altered genes in gastric cancer and continue to refine the molecular landscape.
Getting from types to treatment
Results of molecular classification schema are influenced by input data type and sample size, with estimates that 600 samples per anatomic tumor type are needed for complete characterization (11). Combining genomic data from five previously published datasets Li and colleagues identified six previously unreported significantly mutated genes (SMGs) in regular mutated (RM) gastric cancer (3,5-7,10,12) (Table 1). Consistent with global incidence patterns well over half of the input data were derived from Asian patients (Table 1) (13). To pursue an initial classification scheme, the authors subdivided out 455 RM tumors with a mutation burden averaging around 2.4 mutations per megabase (Mb) pair from 89 hypermutated (HM) tumors with an average of 20.5 mutations/Mb. The classification of tumors into regular and HM signatures led to the observation of mutations at the TpCpW DNA motif (W = A or T; mutated nucleotide underlined) predominating in regular versus HM gastric cancers. Mutations at this motif are typical of the APOBEC cytidine deaminase signature and the authors found a positive correlation with APOBEC3B mRNA levels and TpCpW mutations in the RM subset though not in the HM cancers (14). High levels of microsatellite instability (MSI-H) are found in the HM cohort by Li et al., and define microsatellite instable (MSI) in both the The Cancer Genome Atlas (TCGA) and Asian Cancer Research Group (ACRG) subgroups; however, there is significant variation in mutation count (mutations/DNA Mb) among the non-MSI groups (3,4). Mutations per DNA Mb has been correlated with response to immune checkpoint inhibitors in several tumor types, providing clinical relevance to mutation burden as a gastric cancer stratification factor (15,16).

Full table
Utilizing established computational algorithms, specifically MutSigCV, MutSigCL, and MutSigFN, Li et al. identified 31 SMGs causally linked to tumorigenesis among the 455 RM cohorts. Utilizing their large dataset they observed six previously unreported genes recurrently altered, specifically XIPR2 (7.3%), NBEA (7.0%), COL14A1 (4.4%), AKAP6 (3.7%), CNBD1 (3.1%), ITGAV (3.1%). These genes have been observed to be altered across alternate tumor types, and involved in diverse cellular processes including actin binding, phospholipid binding, poly(A) RNA binding, ion channel binding, and protease binding (10). While none of these genes are established driver alterations, their identification as SMGs in gastric cancer does support the importance of large sample size for sensitive detection of recurrent alterations. Several large series across cancer types have exemplified the use of large genomic datasets to identify uncommon alterations with significant therapeutic implications (17,18).
Interestingly, ERBB2 mutations were found in 3.2% of RM gastric cases from the pooled data, largely consistent with individual prior reports, such as the ACRG in which an ERBB2 mutation rate of 2.8% was reported in microsatellite stable (MSS) tumors (Table 2) (3,4). Of note, the ACRG study also included the 49 patients published by Wong et al. that formed part of the present analysis by Li et al. Owing to chosen methodological approaches by Li and colleagues the therapeutically important rates of ERBB2 amplification are not described. As both TCGA and ACRG included array based somatic copy number analysis, ERBB2 alterations highlight a possible weakness in the study by Li et al., as whole exome sequencing (WES) may not robustly detect gene copy number alterations without specialized computational algorithms. Table 2 highlights the distribution of key genes in gastric cancer across the reported molecular subgroups.
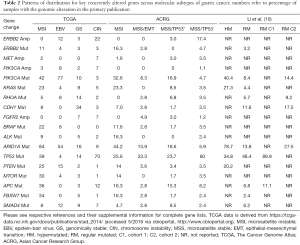
Full table
The confirmation that CDH1 mutations confer a poor prognosis in diffuse type gastric cancer supports the methodology used by Li and colleagues. The mutation rate of 11.6% identified in the combined genomic analysis is consistent with ranges reported by prior studies, though another large series noted the poor prognosis was associated with both intestinal and diffuse subtypes (19). Differing methodological approaches likely account for differing genomic frequencies of CDH1 alterations across gastric cancer studies. Along similar lines the large dataset utilized by Li et al. allowed for employing an unsupervised clustering method to yield separation of RM gastric cancer into two cohorts with differing prognoses. The investigators noted that cohort 1 (C1) showed overlap with TCGA chromosome instability (CIN) subtype while cohort 2 (C2) was evenly distributed among CIN and genomically stable (GS) subtypes and C1 was associated with a longer median survival (roughly 40 months vs. not reached) (10). The study noted that eight differential SMGs (TP53, CDH1, ARID1A, PIK3CA, XIRP2, APC, ERBB2, and RHOA) could retain the prognostic significance of the larger SMG list used to characterize C1 and C2 regular-mutated gastric cancer. Notably, this does differ somewhat from the isolated ACRG study that reported MSS tumors with intact p53 activity (MSS/TP53+) exhibited better survival than MSS tumors with loss of p53 function due to mutation (MSS/TP53−) (4). The ACRG study further classified a MSS/epithelial-to-mesenchymal transition (EMT) subgroup which exhibited the worst survival and contained a relatively high proportion of ARID1A mutations. Though the MSS/EMT subgroup was predominantly composed of Lauren classification diffuse subtype histology with peritoneal spread as the most common pattern of recurrence, CDH1 and RHOA mutations were surprisingly rare preferentially clustering in the MSS/TP53+ group consistent with the C2 classification. Li and colleagues should be commended on their use of large pooled genomic datasets, but the clinical utility of their classification schema requires further prospective study.
The importance of using genomic data to refine tumor classification is well recognized and pioneered by multiple TCGA studies and pan-cancer analyses (20,21). However, establishing and understanding genomically-defined prognostic subgroups has not yet reliably translated to improved patient outcomes in gastric cancer, as these are inherent tumor features not readily modified. We are not aware of prospective therapeutic studies in which molecular subtype (by TCGA or other) has guided treatment in gastric cancer. Several ongoing trials, particularly with immune-mediated therapies, may clarify the predictive ability of mutational signatures in gastric cancer. In a recent prospective trial of the anti-PD-L1 antibody atezolizumab, higher mutational burden was predictive of benefit from treatment in advanced bladder cancer, helping to validate molecular subgroups as predictive biomarkers (22). Significant work is needed before we can confidently say that immune mediated therapies could/should be restricted based on genomic subtype. Interestingly, computational approaches have suggested that a mutational burden of 10 mutations/Mb may be predictive of tumors more likely to harbor malignancy-associated neoantigens, closely paralleling the TCGA cutoffs (11.4 mutations/Mb) and 8.3 mutations/Mb used to separate HM and non-HM gastric cancers by Li and colleagues (3,10,23).
An interesting observation in the study by Li et al. is the breakdown of PIK3CA hotspot alterations by regular and HM gastric cancer. RM gastric cancer showed significant enrichment in helical domain mutations (E542K and E545K) whereas HM tumors contained catalytic domain H1047R alterations (4,10). If APOBEC-mediated processes are the main operative method in RM gastric, and defective DNA proofreading and repair dominate HM tumors, then this observation may have larger implications. How, at the fundamental molecular level, mutagenic exposures select for one activating kinase alteration over another is not well understood and begs further investigation in gastric cancer. The distribution of key genes, several with immediate therapeutic implications, across gastric cancer subtypes are yet to be fully exploited (Table 2). In a smaller series of breast and gynecologic malignancies, the H1047R PIK3CA alteration was associated with a numerically higher response to PI3K pathway inhibitors than non-H1047R PIK3CA mutations, suggesting possible clinical implications for the observation by Li and colleagues (24).
Conclusion and future directions
Where should ongoing genomic and clinical studies in gastric cancer go from here to capitalize on refined molecular classification? The development of model systems (patient derived cell lines, etc.) representative of each genomic subtype will be important to functionally validate alterations described by Li and others. For example, do ERBB2 amplified MSS/TP53− and MSS/TP53+ gastric cancer subtypes have similar response rates to trastuzumab, or do the genomic context and concurrent alterations modify the efficacy? As the data from Li et al. is drawn predominantly from untreated tumors, the direct applicability of this genomic landscape to treatment-refractory metastatic patients is difficult to discern, and research utilizing samples from more stage IV patients, particularly those with prior therapy (chemotherapy, immunotherapy, radiotherapy, and targeted therapy) may add additional information about possible subtype transformation. Studies relying primarily on genomic data by definition offer only a summative and static view of tumors and we anticipate incorporation of more fluid and functional assays will be needed to realize and optimize the use of genomic data (25). More clinical trials stratified based on molecular classifications should be designed to test the anti-tumor efficacy (i.e., mesenchymal vs. non-mesenchymal) to maximize the treatment response for specific target drugs.
The interesting question of whether similar classification schemas can be derived or recapitulated using commercially available hybrid capture based next-generation sequencing assays remains to be determined. Many of these tests incorporate whole exon coverage of 200−400 cancer-associated genes, representing 1−2 Mb of the human genome (3 Gigabases), and have established utility in gastric cancer (26). With improving technological advances, collaborative big data effort, and refined classifications as described by Li et al. we hope our patients will reap the ultimate benefit from these research endeavors.
Acknowledgments
Funding: This work was supported by the National Cancer Institute of the National Institutes of Health (2K12CA001727-21 to JC).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Zhen-Yu Lin, MD (Cancer center, Union hospital, Huazhong University of Science and Technology, Wuhan, China).
Conflicts of Interest: J Chao has received research support from Merck & Co. SJ Klempner has received honoraria from Foundation Medicine, Inc. J Lee has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449-56. [Crossref] [PubMed]
- Chen K, Yang D, Li X, et al. Mutational landscape of gastric adenocarcinoma in Chinese: implications for prognosis and therapy. Proc Natl Acad Sci U S A 2015;112:1107-12. [Crossref] [PubMed]
- Wong SS, Kim KM, Ting JC, et al. Genomic landscape and genetic heterogeneity in gastric adenocarcinoma revealed by whole-genome sequencing. Nat Commun 2014;5:5477. [Crossref] [PubMed]
- Kakiuchi M, Nishizawa T, Ueda H, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet 2014;46:583-7. [Crossref] [PubMed]
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49. [PubMed]
- Hu B, El Hajj N, Sittler S, et al. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol 2012;3:251-61. [PubMed]
- Li X, Wu WK, Xing R, et al. Distinct Subtypes of Gastric Cancer Defined by Molecular Characterization Include Novel Mutational Signatures with Prognostic Capability. Cancer Res 2016;76:1724-32. [Crossref] [PubMed]
- Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014;505:495-501. [Crossref] [PubMed]
- Wang K, Yuen ST, Xu J, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet 2014;46:573-82. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Roberts SA, Lawrence MS, Klimczak LJ, et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet 2013;45:970-6. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850-9. [Crossref] [PubMed]
- Klempner SJ, Bordoni R, Gowen K, et al. Identification of BRAF Kinase Domain Duplications Across Multiple Tumor Types and Response to RAF Inhibitor Therapy. JAMA Oncol 2016;2:272-4. [Crossref] [PubMed]
- Corso G, Carvalho J, Marrelli D, et al. Somatic mutations and deletions of the E-cadherin gene predict poor survival of patients with gastric cancer. J Clin Oncol 2013;31:868-75. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502:333-9. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69-74. [Crossref] [PubMed]
- Janku F, Wheler JJ, Westin SN, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol 2012;30:777-82. [Crossref] [PubMed]
- Friedman AA, Letai A, Fisher DE, et al. Precision medicine for cancer with next-generation functional diagnostics. Nat Rev Cancer 2015;15:747-56. [Crossref] [PubMed]
- Ali SM, Sanford EM, Klempner SJ, et al. Prospective comprehensive genomic profiling of advanced gastric carcinoma cases reveals frequent clinically relevant genomic alterations and new routes for targeted therapies. Oncologist 2015;20:499-507. [Crossref] [PubMed]


