
A novel role for Cish in the inhibition of TCR signaling
Cancer immunotherapeutics focus primarily on stimulating the immune system to elicit endogenous immune responses to fight cancer or transferring primed immune effectors in adoptive cell transfer (ACT) paradigms (1,2). CD8+ T cells are desirable immune effectors in cancer, however, their antitumor activity is often attenuated by tolerance mechanisms or the suppressive nature of the tumor microenvironment (3). As such, the goal of cancer immunotherapies is increasingly focusing on overcoming the tumor tolerance barrier by enhancing the antigen reactivity and effector function of tumor-specific T cells (4), however, the presence of T cells with high-affinity for their cognate antigen is often precluded by thymic selection (5,6). Therefore, developing methods of enhancing T cell receptor (TCR) signaling or functional avidity to maximize antitumor efficacy of tumor-specific T cells (7) can improve cancer therapies employing ACT, vaccines, or checkpoint inhibitors. Current approaches include the development of affinity-enhanced antigen receptors for ACT therapies, but these have been met with challenges (8,9), including treatment-related toxicities (10-12). In an effort to identify new druggable targets to increase CD8+ T cell functional avidity for tumor-specific antigens and increase tumor killing, Palmer et al. identified a novel intrinsic pathway inhibiting TCR signaling cascades and limiting CD8+ T cell activity in murine tumors (13).
There are eight existing members of the suppressor of cytokine signaling (SOCS) family of molecules (SOCS1-7 and Cish), which share a central SH2 domain and a C-terminal SOCS box. SOCS molecules are thought to negatively regulate cytokine signaling by sequestrating downstream signaling components (such as JAKs and STATs) and facilitating their proteasomal degradation using an E3 ligase-like mechanism (14). While it is known that Cish is induced upon TCR stimulation or the addition of cytokines (15,16), it’s precise role in immune regulation has been elusive. Cish has been shown to interact with the IL-2, erythropoietin and growth hormone receptors and is implicated in the inhibition of STAT5 phosphorylation by direct competition for receptor binding sites (17,18). Palmer et al. challenge the current paradigm that Cish regulates immunity primarily by regulating cytokine signaling cascades. Instead, they focused on the role of Cish in TCR signaling in tumor-specific CD8+ T cells.
Using a TCR transgenic model (pmel-1) targeting the melanoma antigen gp100, and a newly-created Cish-deficient mouse model, Palmer et al. explored the function of Cish in effector CD8+ T cells. They detected basal levels of Cish in naïve T cells from wild-type mice that were rapidly induced upon TCR stimulation and enhanced in the hours to follow. They also demonstrated that Cish is up-regulated in an antigen-dependent manner in tumor-specific CD8+ T cells infiltrating melanoma tumors. Deletion of Cish resulted in enhanced CD8+ T cell expansion, functional avidity, and multi-functionality (production of multiple cytokines) in response to in vitro stimulation. Moreover, in vivo studies evaluating antitumor efficacy of Cish-deficient CD8+ T cells demonstrated that the increased expansion and functional activity of these T cells resulted in superior therapeutic antitumor activity, eliminating established tumors. Importantly, to evaluate the therapeutic and translational applicability of their findings, Palmer et al. knocked down Cish expression in patient T cells using a retrovirus encoding a short hairpin microRNA (shmiR) and co-transduced patient T cells with a retrovirus encoding TCRs against various tumor-specific antigens. Analysis of effector function and tumor reactivity recapitulated the observations in Cish-deficient mice, suggesting that Cish negatively regulates both mouse and human TCR signaling and T cell-mediated antitumor immunity.
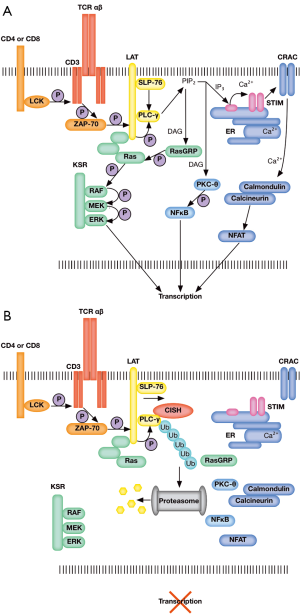
Interestingly, Palmer et al. observed no differences in STAT5 phosphorylation or activation levels between wild-type and Cish-deficient mice, contrary to existing reports. In search of an alternative mechanism by which Cish negatively regulates T cell activation and activity, gene expression profiles where compared between wild-type and Cish−/− CD8+ T cells following TCR activation, revealing the up-regulation of critical pro-functional, proliferative and pro-survival genes (Tbx21, Cmyc, and Bcl2l1, respectively) in Cish−/− T cells. Further, an unexpected physical interaction between Cish and the critical TCR signaling component PLC-γ1 was identified. PLC-γ1 converts PIP3 into IP2 and DAG, increasing calcium flux and the activation of enzymes critical for the transcriptional activation of T cell regulators such as NFAT and NFκB (Figure 1A). Cish targets PLC-γ1 for proteasomal degradation via polyubiquitination, inhibiting downstream TCR signaling, preventing the activation of NFAT and NFκB, and inhibiting T cell activation (Figure 1B). Their findings implicate Cish as an intrinsic TCR checkpoint inhibitor, and position Cish as a novel cancer immunotherapy target to overcome tolerance and enhance T cell functional avidity, immune responses, and antitumor immunity.
However, a few aspects of this study should be addressed. The authors performed studies using T cells derived from germline knockouts. It has been observed previously, that aged, germline Cish knockout mice developed lung inflammation that did not occur in animals with T cell lineage-specific Cish deletion (19). Thus, it’s possible that total-body deletion of Cish might impact T cell development/function in a T cell extrinsic manner. Further, the authors reported the absence of immunopathology in unmanipulated Cish−/− mice, however, the translational application of these findings is questionable. In a clinical setting, where cancer patients are likely immunocompromised, and infections are encountered at a frequency higher than those in the pathogen-free conditions in a mouse facility, autoimmunity might be a consequence of targeting Cish. The absence of Cish is also reported to induce TCR-dependent hyperactivity, which might cause spontaneous TCR signaling or T cell exhaustion, especially in the context of T cells with natural TCRs. The potential for autoimmunity and TCR hyperactivity by implementing Cish-depletion/inhibition to treat cancer will require characterization in future studies.
In conclusion, Palmer et al. identified a novel role for Cish in immune regulation, and provided sufficient evidence supporting Cish as an inhibitor of TCR signaling with therapeutic potential. They demonstrated that Cish elimination enhanced T cell functionality and antitumor immunity. While, further studies are needed to develop Cish-targeted immunotherapeutic strategies, the current study suggests that Cish inhibitors may enhance the efficacy of many current cancer immunotherapies, including adoptive cell therapy with TCR or CAR-expressing T cells, cancer vaccines and checkpoint inhibitors, by improving TCR signaling within the tumor microenvironment.
Acknowledgments
Funding: The authors were supported by the Margaret Q. Landenberger Research Foundation and the W.W. Smith Charitable Trust.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chen Qian (Center for Inflammation & Epigenetics, Houston Methodist Hospital Research Institute, Houston, USA).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.05.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vatakis DN, Koya RC, Nixon CC, et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc Natl Acad Sci U S A 2011;108:E1408-16. [Crossref] [PubMed]
- Ghorashian S, Veliça P, Chua I, et al. CD8 T cell tolerance to a tumor-associated self-antigen is reversed by CD4 T cells engineered to express the same T cell receptor. J Immunol 2015;194:1080-9. [Crossref] [PubMed]
- Jackson SR, Yuan J, Teague RM. Targeting CD8+ T-cell tolerance for cancer immunotherapy. Immunotherapy 2014;6:833-52. [Crossref] [PubMed]
- Makkouk A, Weiner GJ. Cancer immunotherapy and breaking immune tolerance: new approaches to an old challenge. Cancer Res 2015;75:5-10. [Crossref] [PubMed]
- McCaughtry TM, Hogquist KA. Central tolerance: what have we learned from mice? Semin Immunopathol 2008;30:399-409. [Crossref] [PubMed]
- Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol 2012;13:121-8. [Crossref] [PubMed]
- Oren R, Hod-Marco M, Haus-Cohen M, et al. Functional comparison of engineered T cells carrying a native TCR versus TCR-like antibody-based chimeric antigen receptors indicates affinity/avidity thresholds. J Immunol 2014;193:5733-43. [Crossref] [PubMed]
- Zhu Z, Singh V, Watkins SK, et al. High-avidity T cells are preferentially tolerized in the tumor microenvironment. Cancer Res 2013;73:595-604. [Crossref] [PubMed]
- Chung B, Stuge TB, Murad JP, et al. Antigen-specific inhibition of high-avidity T cell target lysis by low-avidity T cells via trogocytosis. Cell Rep 2014;8:871-82. [Crossref] [PubMed]
- Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006;314:126-9. [Crossref] [PubMed]
- Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009;114:535-46. [Crossref] [PubMed]
- Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013;122:863-71. [Crossref] [PubMed]
- Palmer DC, Guittard GC, Franco Z, et al. Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance. J Exp Med 2015;212:2095-113. [Crossref] [PubMed]
- Trengove MC, Ward AC. SOCS proteins in development and disease. Am J Clin Exp Immunol 2013;2:1-29. [PubMed]
- Li S, Chen S, Xu X, et al. Cytokine-induced Src homology 2 protein (CIS) promotes T cell receptor-mediated proliferation and prolongs survival of activated T cells. J Exp Med 2000;191:985-94. [Crossref] [PubMed]
- Jin P, Wang E, Provenzano M, et al. Molecular signatures induced by interleukin-2 on peripheral blood mononuclear cells and T cell subsets. J Transl Med 2006;4:26. [Crossref] [PubMed]
- Endo T, Sasaki A, Minoguchi M, et al. CIS1 interacts with the Y532 of the prolactin receptor and suppresses prolactin-dependent STAT5 activation. J Biochem 2003;133:109-13. [Crossref] [PubMed]
- Landsman T, Waxman DJ. Role of the cytokine-induced SH2 domain-containing protein CIS in growth hormone receptor internalization. J Biol Chem 2005;280:37471-80. [Crossref] [PubMed]
- Yang XO, Zhang H, Kim BS, et al. The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation. Nat Immunol 2013;14:732-40. [Crossref] [PubMed]


