Gut microbia dysbiosis in non-alcoholic fatty liver disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) represents the spectrum of fatty liver that ranges from simple steatosis to steatohepatitis leading to fibrosis and cirrhosis. NAFLD is often considered the hepatic manifestation of metabolic syndrome and it is closely associated with diabetes, insulin resistance and obesity. Prevalence of NAFLD ranges from 10–35% and the differences in prevalence are due to differences in diagnostic methods used to diagnose NAFLD, as well as differences in NAFLD prevalence amongst various ethnic groups. However, the progression of non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatits (NASH) is seen in 3–5% of the population, and this subgroup is at a high risk of progression to advanced fibrosis including cirrhosis and hepatocellular carcinoma (HCC) (1,2). There is increasing evidence that gut microbiota plays a role in the development of numerous diseases, and thus gut dysbiosis has been implicated in obesity (3,4) cardiovascular disease (5), diabetes (6,7) and metabolic syndromes (8,9) including non-alcoholic fatty liver and NASH (10-12). The study by Boursier et al. (13) attempts to link gut dysbiosis with severity of fatty liver disease, finding certain microbial subgroups associated with different stages of NAFLD including NASH and level of fibrosis.
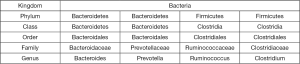
The authors evaluated 57 NAFLD patients, 30 of whom had F0-F1 liver fibrosis at liver biopsy (10 with NASH) and 27 patients with fibrosis stage F >2 (25 with NASH) at liver biopsy. The liver biopsy was assessed by a blinded liver pathologist from NASH Clinical Research Network. The stool samples were collected at the time of liver biopsy and sequenced and analyzed for classification of gut microbiome using 16S ribosomal RNA gene sequencing. Patients were stratified into three groups: NAFLD without NASH with F0/F1 fibrosis, NASH with F0/F1 fibrosis and NAFL D with (25 patients) or without (2 patients) NASH with F >2 liver fibrosis. There were 12 bacteria phylum, 65 family types and 133 genera in the stool samples; at the phylum level of classification, there were no differences in the gut microbiome between the NAFLD and NASH groups, but at the genus level there was a statistically significant increase in Bacteroides and lower abundance of Prevotella (Figure 1), two species which are in an inverse relationship when patients progressed from NAFL to NASH, even when adjusting for metabolic factors such as metabolic syndrome, BMI, diabetes, lipids and hypertension. Furthermore, Boursier et al. found a significant difference between the gut microbial of patients with F0/F1 fibrosis compared to those with F ≥2 fibrosis, with respect to three genera: Bacteroides, Prevotella and Ruminococcus. Those with F ≥2 fibrosis had higher abundances of Ruminococcus and Bacteroides and lower amounts of Prevotella. In addition, Ruminococcus counts were independently associated with fibrosis score ≥2. The author’s concluded that analysis of gut microbial is predictive of NAFLD severity, and may be involved in the pathogenesis of NAFLD including progression to NASH and advanced fibrosis.
Previous studies based on human microbiota also demonstrated the association between gut microbiota and different NAFLD phenotypes, ranging from simple steatosis to NASH. However, there were several differences in the results, and the level of taxonomy examined (e.g., phylum v. genus) often varied (Figure 1). In studies by Mouzaki et al. (14), Zhu et al. (15), and Wong et al. (16), biopsy proven NASH patients had stool examined for dysbiosis of gut microbiota.
Mouzaki et al. (14) compared the gut microbiota of 50 patients with NAFL (simple steatosis, n=11), NASH (n=22) and healthy controls (n=17) established though liver biopsy. Differences in age, gender and BMI were present in the groups at baseline, and degree of liver fibrosis was not stated. This study found higher fecal Clostridium cocoides levels in patients with NASH compared to simple steatosis, though this difference disappeared when controlled for BMI and recent fat intake. Percentage of the class Bacteroidetes (containing both Bacteroides and Prevotella) was significantly lower in patients with NASH compared to both simple steatosis and health controls, even when controlling for BMI and fat intake. This was one of the first studies to link gut dysbiosis and NAFLD, and evaluated gut microbial at the level of class, but not genus as was the case in the Boursier et al. article, which limits comparisons between these two studies. This study also did not control for other metabolic factors as was done in the Boursier study, which may have influenced the outcomes as well. Additionally, this study was also limited by small sample size, large differences in the comparison groups at baseline, and lack of racial diversity of the study participants.
Zhu et al. (15) evaluated and compared gut microbial composition in 63 children and adolescents (NASH vs. obese vs. normal control) defined as having NASH on liver biopsy (n=22), obese without NASH (BMI >95th percentile with normal liver enzymes, n=25), or healthy controls (BMI <85th percentile, n=16). Authors reported that the obese and NASH subjects had more similar microbiomes to each other than the healthy control group. Contrary to the Mouzaki et al. study, Zhu et al. found a higher number of Bacteroides and decrease in Firmicutes in the obese and NASH groups, compared to the healthy control group. Prevotella was markedly more abundant in the NASH and obese groups compared to the healthy group (Figure 1). This is a contrast to the Boursier study which found Provotella in lower numbers in the group which progressed to NASH or fibrosis.
Wong et al. (16) evaluated 16 biopsy proven NASH, and 22 healthy controls with normal liver function tests who had no history of liver disease, aged 24–69. The primary goal of this study was to evaluate the effect of probiotics on gut microbiota . However, baseline data were analyzed for the gut microbiota of participants and found that at the phylum level, Bacteroidetes was found to be most abundant type of microbe in both NASH and healthy controls, but Firmicutes abundance was increased in healthy patients compared to NASH at baseline, similar to results from Mouzaki et al. The order Aeromonadales, families Succinivibrio and Porphyromonadaceae and genera Parabacteroides and Allisonella were more abundant in NASH patients than healthy controls, and the order Clostridiales and genera Faecalibacterium and An aero sporobacter were less abundant in NASH patients. The limitations of this study are that no liver biopsy was done on “healthy controls”, they were assumed to be free of NAFLD because liver enzymes were normal. This study analyzed bacteria at many levels of the taxonomical classification (i.e., Phylum, class, order and genus) and this makes comparison of results with other studies difficult.
Conclusions
Overall, Boursier et al. did an excellent study correlating gut microbial composition with NAFLD spectrum severity but there are several limitations of this study. While the authors found the only limitation of this study to be sample size, this study is also limited by the composition of the sample which is predominately male, and age above 50. This study was also conducted amongst people of European descent, limiting applicability to persons of other racial origin. Furthermore, this study not only lacks healthy control patients without NAFLD, but also without age, sex, race and co-morbid condition matches, which would present a more robust study. It also did not attempt to control for diet or medications [including probiotics, proton pump inhibitors (PPI), antibiotics] of the participants, which may influence gut microbiota composition. This study also does not consider the differences in gut microbiota amongst subjects of different ages, which has previously been characterized (17) or consider in more detail the different levels of advanced fibrosis above F ≥2, as persons with cirrhosis has been seen to have a significantly different microbial profile than healthy controls (18,19). Though all of the studies cited found differences in gut microbiota based on NAFLD spectrum severity, the correlation of which bacteria is associated with NASH is contradictory or difficult to compare, as not all studies compare data at the same bacterial classification, and sequencing of gut microbiota was not standardized (20). The Boursier et al. study does not address these differences in results, and standardization of the approaches for characterizing the gut microbiota may be useful for application of study results in the future. Boursier et al.’s decision to characterize the differences in gut microbiota at the genus level should be commended, as more specific levels of categorization of gut microbiota are more useful than more general categorization (e.g., at the level of phylum) for understanding the role of gut microbiota in the pathogenesis of disease and studying interventions (i.e., pre and probiotics) for intervention in disease progression.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Anqiang Wang (Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.07.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274-85. [Crossref] [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005-23. [Crossref] [PubMed]
- Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 2009;457:480-4. [Crossref] [PubMed]
- Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol 2010;26:5-11. [Crossref] [PubMed]
- Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57-63. [Crossref] [PubMed]
- Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 2010;5:e9085 [Crossref] [PubMed]
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012;490:55-60. [Crossref] [PubMed]
- Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 2009;15:1546-58. [Crossref] [PubMed]
- Murphy EF, Cotter PD, Hogan A, et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut 2013;62:220-6. [Crossref] [PubMed]
- Le Roy T, Llopis M, Lepage P, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013;62:1787-94. [Crossref] [PubMed]
- Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482:179-85. [PubMed]
- De Minicis S, Rychlicki C, Agostinelli L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology 2014;59:1738-49. [Crossref] [PubMed]
- Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016;63:764-75. [Crossref] [PubMed]
- Mouzaki M, Comelli EM, Arendt BM, et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013;58:120-7. [Crossref] [PubMed]
- Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013;57:601-9. [Crossref] [PubMed]
- Wong VW, Tse CH, Lam TT, et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis--a longitudinal study. PLoS One 2013;8:e62885 [Crossref] [PubMed]
- Claesson MJ, Cusack S, O'Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A 2011;108:4586-91. [Crossref] [PubMed]
- Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59-64. [Crossref] [PubMed]
- Liu J, Wu D, Ahmed A, et al. Comparison of the gut microbe profiles and numbers between patients with liver cirrhosis and healthy individuals. Curr Microbiol 2012;65:7-13. [Crossref] [PubMed]
- Tedjo DI, Jonkers DM, Savelkoul PH, et al. The effect of sampling and storage on the fecal microbiota composition in healthy and diseased subjects. PLoS One 2015;10:e0126685 [Crossref] [PubMed]