
GD3 mediated immune response via vascular endothelial growth factor in ovarian cancer
Introduction
Cancer constitutes a major public health problem and one of the most frequent causes of death in the Western world. It has been reported that 1,685,210 new cancer cases and 595,690 deaths from cancer are estimated to occur in the United States in 2016 (1). The economic burden of cancer is associated with expenditures including aspects such as prevention, screening and treatment services and the lost productivity due to cancer-related death (2). Furthermore it has been estimated that by 2030, the number of new cases in various types of the disease will reach 23 million per year corresponding to an increase of 70% when compared to relevant data from 2012 (3).
Ovarian cancer is the most lethal gynecological type of cancer in developed countries. Approximately 23,000 women will be diagnosed with ovarian cancer in the forthcoming years, according to SEER data and about 15,000 of them will die of the disease (1). Ovarian cancer lethality derives from the fact that the majority of ovarian cancer sufferers report the disease when in advanced stages (4). Treatment of advanced disease involves cytoreductive surgery combined with carboplatin/paclitaxel chemotherapy. Despite the initial effectiveness of this therapeutic approach, the majority of women will relapse, with a median PFS of around 18 months, and eventually die from ovarian cancer (4). The necessity for novel therapies, therefore, is obvious. Recent research has been focused on targeted therapies, i.e. treatment targeting biologically relevant molecular factors, present in the tumor or its microenvironment. Such targets are constantly sought in order to extend the already growing pipeline of therapies by seeking effective ways to combat the disease including circulating tumor cells, soluble tumor markers and the use of genomic or proteomic information (5,6).
Angiogenesis is a process that refers to the formation of new blood vessels, and it constitutes a hallmark process of cancer progression and metastasis. The angiogenetic process is rather complex and involves a large number of cytokines and associated receptors and it is one of the most prominent processes in cancer related to tumor survival and induction of tumor metastasis (7). It is a dynamic process with pro-angiogenic and anti-angiogenic proteins involved in its regulation. Angiogenesis plays a major role in tumorigenesis, tumor expansion and ascites formation in ovarian cancer. Ascitic fluid represents an easily accessible biological fluid compared to tumor samples, and it may be more representative of the biological behavior of ovarian cancer compared with blood (8).
The vascular endothelial growth factor (VEGF) is one of the most potent pro-angiogenic factors and it may be associated with poor prognosis and resistance to therapy in ovarian cancer (9). VEGF levels have also been shown to be higher within ascitic fluid when compared to blood, suggesting that the tumor burden is higher in the peritoneal cavity, which is the anatomic region with the highest disease impact.
Except angiogenesis, VEGF has been implicated in a series of other processes that may be associated with ovarian cancer and moreover it may be directly interacting with the patients’ immune system in a number of ways. More specifically, we have shown that VEGF may be suppressing T cell activation and proliferation in both blood and in ascites of ovarian cancer patients (10,11). Other groups have shown that VEGF may be involved in immune system suppression in ways such as via the downregulation of dendritic cells (12,13), and via the attraction and accumulation of immunoregulatory cells such as myeloid-derived suppressor cells (MDSCs) affecting T cells but also natural killer (NK) cells (14,15). MDSCs comprise a heterogeneous group of cells of myeloid origin, one that includes a variety of cells such as myeloid progenitor cells and also immature myeloid cells. The latter includes granulocytes, and dendritic cells that show significant immunosuppressive properties. MDSC accumulation in the tumor microenvironment leads to suppression of T-cell response in a number of ways and VEGF-A is responsible for the accumulation of MDSC.
T regulatory cells (T regs) may also be accumulated by VEGF and other pro angiogenic factors such as the placental growth factor (PIGF) (16) via the implication of cytokines such as tumor growth factor beta (TGF-β) but also interleukin 10 (IL-10). VEGF may also affect accumulation of tumor associated macrophages (17). In this case VEGF is not the only player but its effects are also assisted by the expression and secretion of other cytokines such as IL-10 and IL-4. VEGF can also induce the expression of Fas ligand, a well known driver of apoptosis of T cells, on endothelial cells, which have specifically acquired the ability of preferentially killing cytotoxic CD8+ T cells and not affecting T regs (18).
For that matter VEGF has been a target of research in all types of cancer, including ovarian cancer in order to establish therapies that may interfere with its function and prevent tumor spread. Antibody based therapy has been introduced in the clinic with encouraging results when Bevacizumab/Avastin and also anti-angiopoietin agents have been used (19).
The paper by Tiper et al., accepted and currently in press at the journal of Clinical Cancer Research, deals though with another aspect of VEGF mediated suppression of the immune system in ovarian cancer (20). In this study, VEGF is shown to potentiate immune suppression by human ovarian cancer cells via GD3 ganglioside. This work builds on previous studies by the same group that have exhibited the presence of GD3 in ascitic fluid from ovarian cancer patients and in addition they have shown that NKT cells’ antitumor responses may be hindered (21). Interestingly the cross talk between VEGF and GD3 has been reported in the past in the case of another type of cancer, namely glioma, where GD3 was shown to be a potent stimulator of VEGF release (22).
The authors have chosen to work with ascitic fluid rather than blood in this study and in our opinion, this is a well-driven decision since ascitic fluid contains numerous angiogenesis related proteins in higher amounts than blood, including VEGF. In their latest study (20) they have focused in the effects that this mechanism of tumor immunity evasion may achieve targeting NKT cells as the end point of suppression. For that matter conditioned media from ovarian cancer cell lines has been shown to inhibit CD1d mediated NKT cell activation.
We believe that showing a similar effect on other immune cell populations would have been of interest too, especially in the ratio of CD4+/CD8+ T cells and also Tregs. These populations are of great interest, alongside NK and NKT cells of course, since CD8+ cytotoxic T cells have long been studied in various types of cancer for the effects that may exert on the tumor and Tregs function has been the subject of a great debate as to whether their presence in the tumor can act as a prognostic factor in terms of both progress free survival (PFS) and overall survival (OS) of the patients (23). Our notion is strengthened by the fact that they did find tumor infiltration by immune cells although they clearly state that there was a higher percentage of CD4− CD8− cells present in NKT cells inhibitory ascites rather than the opposite. Nevertheless the differences in these cell populations might have been of interest and this may be the aim of future work.
In accordance with previous findings (8,9) the authors have measured high VEGF levels in patients’ ascitic fluids and also in the supernatants of cultured ovarian cancer cell lines. This is further in concert with our previous studies in terms of the levels of VEGF addressed in ascitic fluids showing once more the validity of using this biological material in this type of research. They also used Bevacizumab and Genistein in order to abrogate the effects of VEGF and screened for NKT cell activation. Treatment of ovarian cancer cell lines with both these agents resulted in VEGF inhibition as expected and this had as a downstream effect the restoration of NKT cell activity. Similar effects have been reported regarding the restoration of function of dendritic cells upon VEGF blockage with anti-VEGF agents including Bevacizumab (24). Moreover, regarding the relationship between VEGF overexpression and NKT population cell suppression, this has been shown by other groups in the past including our own (9). In this paper by Bamias et al., VEGF levels were inversely correlated with NKT-like cells (CD3+ CD56+) and these were also correlated to platinum resistance. CD3+ CD56+ cells are a well-defined and important population regarding tumor rejection, since they can mediate non-MHC-restricted cytotoxicity, while previous experiments using blood cells from patients with lymphomas have shown a rather significant expansion of this population in ex vivo conditions and have also shown considerable in vitro cytotoxicity (9). Therefore the results presented by Tiper et al., are in accordance with previous studies that do show similar effects of VEGF on immune cell populations.
The novelty of the paper by Tiper et al., is the addition of GD3 in this mechanism of immunosuppression. The same group has reported previously that GD3 that is present in ascitic fluid shows potential in inhibiting anti-tumor NKT cell responses and higher levels of GD3 have been shown to be present in the blood of ovarian cancer patients when compared to healthy donors as a result of ganglioside shedding from the surface of tumor cells. The authors performed further work in order to show that GD3 inhibition is responsible for restoring NKT cell function by overexpressing the membrane associated NEU3, a molecule that has been shown to decrease GD3 (20). What is of note here is that the source of GD3 seems to play an important role in the functional impact of GD3, with the GD3 derived from bovine brain seeming to be more effective than the one derived from buttermilk for example. Why is there a difference in their function depending on the origin of the molecule is something that poses questions about the establishment of functionality of GD3 topologically.
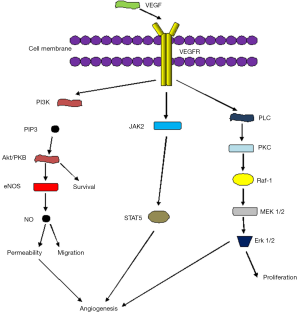
The authors have also shown that the incubation of ascites from patients with target cells activates different proteins in the Map Kinase Pathway (MAPK) including molecules such as ERK and p38. The interesting issue here is that ascites from different patients do not show a uniform pattern of induction of proteins related to the MAPK pathway but in fact they exhibit differences in the repertoire of molecules expressed. The MAPK pathway is activated as a downstream effect of the VEGF signalling cascade (7) (Figure 1) and since the ascitic fluids contain high amounts of VEGF, the activation of this pathway is highly expected to occur. The difference in the expression of molecules from the MAPK pathway depending on the ascites used is of interest but surely a higher number of samples may be necessary to investigate this further. To enhance the importance of the MAPK pathway, in the most recent study that our group has published, a MAPK functionally related protein, MIP-1, is shown to be a part of a proposed prognostic signature model that may be used to select patients for anti angiogenic therapies (25).
Conclusions
The necessity for novel therapies in ovarian cancer is growing. Targeting pro angiogenic factors with VEGF being the most prominent target has shown considerable promise and has already been incorporated into the current treatment paradigm for ovarian cancer. In the paper by Tiper et al., the authors describe the cross talk between VEGF and the ganglioside GD3 as a means for the tumor to evade the patient’s immune system. More specifically, they have found that VEGF suppresses GD3 and have hypothesized that activation of MAPK kinase pathway components such as ERK induce the ganglioside shedding by ovarian cancer cells. They have also found that VEGF blockade via the use of Bevacizumab may restore NKT cell responses.
This proposed mechanism of VEGF suppressing the immune system, mainly acting on the innate immune cell fortress, is being added to the ways proposed so far for VEGF’s role in the immune system evasion. Given these encouraging results we are looking forward to new data on the subject that will broaden the target repertoire for battling this devastating disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Da Li (Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.07.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Yabroff KR, Lund J, Kepka D, et al. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev 2011;20:2006-14. [Crossref] [PubMed]
- Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol 2012;13:790-801. [Crossref] [PubMed]
- Sopik V, Iqbal J, Rosen B, et al. Why have ovarian cancer mortality rates declined? Part I. Incidence. Gynecol Oncol 2015;138:741-9. [Crossref] [PubMed]
- Obermayr E, Castillo-Tong DC, Pils D, et al. Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance -- a study of the OVCAD consortium. Gynecol Oncol 2013;128:15-21. [Crossref] [PubMed]
- Taylor DD, Gercel-Taylor C, Parker LP. Patient-derived tumor-reactive antibodies as diagnostic markers for ovarian cancer. Gynecol Oncol 2009;115:112-20. [Crossref] [PubMed]
- Gavalas NG, Liontos M, Trachana SP, et al. Angiogenesis-related pathways in the pathogenesis of ovarian cancer. Int J Mol Sci 2013;14:15885-909. [Crossref] [PubMed]
- Bamias A, Tsiatas ML, Kafantari E, et al. Significant differences of lymphocytes isolated from ascites of patients with ovarian cancer compared to blood and tumor lymphocytes. Association of CD3+CD56+ cells with platinum resistance. Gynecol Oncol 2007;106:75-81. [Crossref] [PubMed]
- Bamias A, Koutsoukou V, Terpos E, et al. Correlation of NK T-like CD3+CD56+ cells and CD4+CD25+(hi) regulatory T cells with VEGF and TNFalpha in ascites from advanced ovarian cancer: Association with platinum resistance and prognosis in patients receiving first-line, platinum-based chemotherapy. Gynecol Oncol 2008;108:421-7. [Crossref] [PubMed]
- Ziogas AC, Gavalas NG, Tsiatas M, et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer 2012;130:857-64. [Crossref] [PubMed]
- Gavalas NG, Tsiatas M, Tsitsilonis O, et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer 2012;107:1869-75. [Crossref] [PubMed]
- Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 1996;2:1096-103. [Crossref] [PubMed]
- Dikov MM, Ohm JE, Ray N, et al. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol 2005;174:215-22. [Crossref] [PubMed]
- Zea AH, Rodriguez PC, Culotta KS, et al. L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol 2004;232:21-31. [Crossref] [PubMed]
- Hoechst B, Voigtlaender T, Ormandy L, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009;50:799-807. [Crossref] [PubMed]
- Terme M, Pernot S, Marcheteau E, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 2013;73:539-49. [Crossref] [PubMed]
- Hanada T, Nakagawa M, Emoto A, et al. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol 2000;7:263-9. [Crossref] [PubMed]
- Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med 2014;20:607-15. [Crossref] [PubMed]
- Bamias A, Pignata S, Pujade-Lauraine E. Angiogenesis: a promising therapeutic target for ovarian cancer. Crit Rev Oncol Hematol 2012;84:314-26. [Crossref] [PubMed]
- Tiper IV, Temkin SM, Spiegel S, et al. VEGF Potentiates GD3-Mediated Immunosuppression by Human Ovarian Cancer Cells. Clin Cancer Res 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Webb TJ, Li X, Giuntoli RL 2nd, et al. Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res 2012;72:3744-52. [Crossref] [PubMed]
- Koochekpour S, Pilkington GJ. Vascular and perivascular GD3 expression in human glioma. Cancer Lett 1996;104:97-102. [Crossref] [PubMed]
- Halvorsen EC, Mahmoud SM, Bennewith KL. Emerging roles of regulatory T cells in tumour progression and metastasis. Cancer Metastasis Rev 2014;33:1025-41. [Crossref] [PubMed]
- Gabrilovich DI, Ishida T, Nadaf S, et al. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res 1999;5:2963-70. [PubMed]
- Trachana SP, Pilalis E, Gavalas NG, et al. The Development of an Angiogenic Protein "Signature" in Ovarian Cancer Ascites as a Tool for Biologic and Prognostic Profiling. PLoS One 2016;11:e0156403 [Crossref] [PubMed]


