
Therapeutic potential of Ginsenoside Rg3 via inhibiting Notch/HES1 pathway in lung cancer cells
Introduction
Lung cancer, which is aggressive and malignant, has one of the highest mortality rates in the world. Lung cancer has two major classes according to its biology, prognosis and therapy: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC which accounts for about 85% cases of lung cancer includes two major types: non squamous carcinoma (including adenocarcinoma, large cell carcinoma, other cell types) and squamous cell carcinoma. These cases usually are diagnosed in the late stages of the disease and can then receive only chemotherapy because surgery is not an option and the prognosis is especially poor. The efficacy of chemotherapy has often been limited to the severe chemotherapy-related side effects. Hence, new agents which could be effectively administered without severe adverse effect are urgently needed. Previous studies have reported that natural products such as steroidal saponin are suitable alternatives for developing antitumor drugs (1-6).
Ginsenoside Rg3 is a steroidal saponin extracted from Panax ginseng which is an herbal medicine and is used widespread. It is reported to have various pharmacological effects such as anti-tumor, anti-oxidant, anti-inflammation properties, and angiogenesis (7-11). Numerous cases have been reported that Ginsenosides possessed anti-tumor effects against cancers, such as hepatocellular carcinoma, glioblastoma, esophageal cancer, gastric cancer, pancreatic cancer, ovarian cancer and so on (12-18).
Rg3 has specific effects on tumor growth suppression, including metastasis and angiogenesis inhibition, enhancing the chemosensitivity and radiosensitivity, reversing multidrug resistance, which could be regarded as a novel antitumor agent (19-21). Rg3 has potential antitumor effects based on its inhinition of invasion, metastasis and growth of neovascularization, but the exact mechanism is still unclear.
In attempting to further elucidate the antitumor mechanisms by which Ginsenoside Rg3 works in lung cancer, the cell proliferation, apoptosis and Notch pathway in lung cancer cell lines were evaluated.
Methods
Chemicals
Ginsenoside Rg3 (C42H72O13) was purchased from the Zhejiang Institute for Food and Drug Control (batch No. 110804, Hangzhou, China) and was dissolved in dimethyl sulfoxide (DMSO), then diluted in RPMI 1640 to achieve the final concentration. RPMI 1640 and fetal calf serum was obtained from Hyclone Co. (Logan, UT, USA). FITC Annexin V Apoptosis Detection kit was obtained from BD Biosciences (NJ, USA). Antibodies: Notch1 (C37C7) Rabbit mAb #3439, Notch2 (8A1) Rabbit mAb #2420, hairy and enhancer of split 1 (HES1) (D6P2U) Rabbit mAb #11988, p38 MAPK (D13E1) Rabbit mAb #8690, Caspase-3 (8G10) Rabbit mAb #9665, Caspase-9 (C9) Mouse mAb #9508 at 1:1,000 dilution(Cell Signaling Technology, Danvers, MA, USA).
Cell lines and culture
The human lung cancer cell lines NCI-H1650 (adenocarcinoma; non-small cell), H520 (squamous cell carcinoma; non-small cell) and H1963 (SCLC) were obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA), and were cultured in RPMI 1640 with 10% fetal bovine serum at 37 °C in a 5% CO2 humidified atmosphere.
Cell proliferative inhibition assay
The cell proliferative inhibition of Ginsenoside Rg3 in lung cancer cell lines was detected by MTT assay. NCI-H1650, H520 and H1963 cell lines were seeded respectively. The control group with no treatment was established and each group had triplicate treatments. Then cells were exposed to Ginsenoside Rg3 at various concentrations (1, 5, 10, 20, 40, 80, 160 μmol/L) for 24, 48, and 72 h, with dose- and time-dependent curves generated respectively.
Apoptosis analysis
Annexin-V/PI assay was used to examine cell apoptosis rates. NCI-H1650, H520 and H1963 cell lines were seeded respectively and then treated with Ginsenoside Rg3 (20 μmol/L) and incubation for 24 and 48 h. Cells were collected and examined by using an Annexin V-FITC Apoptosis Detection kit. Cells were stained by Annexin V-FITC and PI, then analyzed by FACSCalibur flow cytometry (BD Biosciences).
Western blot analysis
Following incubation with Ginsenoside Rg3 (20 μmol/L) for 48 h, NCI-H1650, H520 and H1963 cell lines were collected. After cell lysing, equal amounts of protein were electrophoresed and then transferred to polyvinylidene fluoride membranes (Thermo Fisher Scientific Inc., Waltham, MA, USA). Then primary antibodies [Notch1, Notch2, HES1, p38 MAPK, Caspase-3, Caspase-9 and GAPDH antibody (1:1,000)] were incubated with membranes, and then the HRP conjugated secondary antibody (1:10,000). The membranes were visualized using an enhanced chemiluminescence system (Santa Cruz Biotechnology Inc.).
Statistical analysis
Data were presented as means ± S.D. and statistically analysed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Groups were compared by one-way ANOVA test and SNK-q test considering P<0.05 as a significance level.
Results
Effect of Ginsenoside Rg3 on cytotoxicity in lung cancer cells
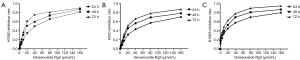
Ginsenoside Rg3 inhibited the growth of NCI-H1650, H520 and H1963 cell lines in a time and dose-dependent manner for 24, 48 and 72 h (Ginsenoside Rg3 concentrations from 1 to 160 μg/mL) in Figure 1A,B,C.
Effect of Ginsenoside Rg3 on apoptosis in lung cancer cells
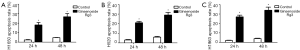
Annexin-V/PI method was used to explore the ability of Ginsenoside Rg3 to induce apoptosis in lung cancer cell lines. Ginsenoside Rg3 induced a significant apoptosis in NCI-H1650, H520 and H1963 cell lines for 24 and 48 h. The apoptosis rates were 2.36%±0.47% in the control group, 18.15%±2.58% in the Ginsenoside Rg3 group for 24 h, and 4.77%±0.22% in the control group, 26.8%±3.43% in the Ginsenoside Rg3 group for 48 h in NCI-H1650 cell ine (Figure 2A). The apoptosis rates were 3.27%±0.51% in the control group, 21.44%±1.36% in the Ginsenoside Rg3 group for 24 h, and 6.53%±0.82% in the control group, 29.56%±2.89% in the Ginsenoside Rg3 group for 48 h in NCI-H520 cell ine (Figure 2B). The apoptosis rates were 2.68%±0.32% in the control group, 27.74%±1.85% in the Ginsenoside Rg3 group for 24 h, and 4.77%±0.45% in the control group, 34.83%±3.38% in the Ginsenoside Rg3 group for 48 h in NCI-H1963 cell ine (Figure 2C).
Effect of Ginsenoside Rg3 on Notch/HES1 and apoptosis pathway in lung cancer cells
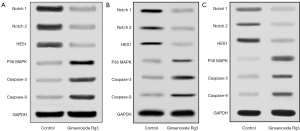
The protein expression levels of Notch/HES1 and apoptosis pathway were evaluated by western blotting analysis. The level of Notch1, Notch2 and HES1 protein expressions decreased, while the level of p38 MAPK, Caspase-3 and Caspase-9 protein expressions increased after Ginsenoside Rg3 treatment (Figure 3A,B,C). Above result suggested decreased Notch1, Notch2, HES1 expressions and increased p38 MAPK, Caspase-3, Caspase-9 expressions contributed to lung cancer cell proliferative inhibition and apoptosis inducing by Ginsenoside Rg3.
Discussion
The Notch signaling pathway is critical for cell fate specification, and it is a signal transduction network that evolutionally conserved. Notch signaling is frequently deregulated in solid tumors including NSCLC, and plays an important role in cell proliferation and apoptosis. Myc, p21, HES and so on are the target genes of Notch. NSCLC patients who carried activated Notch1 were associated with poor survival (22). Overexpression of Notch or HES1 is associated many cancers, such as ovarian cancer, breast cancer, cervical cancer, prostate cancer, colon cancer and NSCLC (23,24). The downstream target of the Notch signaling pathway is HES1. Notch-independent HES1 expression can also result from Hedgehog and c-Jun N-terminal kinase (JNK) signaling as well as from RAS/MAPK signaling (25,26). P38 MAPK and caspase family are critical in the process of cell apoptosis, proliferation and differentiation.
Several studies have reported that a high percentage of lung cancer lines express Notch receptors and their target genes, such as Jagged1, HES1 and Hey1. The inhibition of Notch signaling can reduce tumor cell proliferation (27). Notch signaling is altered in many lung cancers, and the deregulation of the Notch pathway may be a frequent event in NSCLC (22). The upregulation of Notch signaling can increase survivin expression and contribute to lung cancer clonogenic capacity in vitro (28,29). Furthermore, the downregulation of Notch signaling inhibits the growth, migration, and invasiveness of cancer cells and induces cell apoptosis (30). Blocking the Notch pathway can inhibit the growth of lung cancer cells, induce apoptosis and sensitize to chemotherapy (31).
Numerous studies showed that Rg3 had anticancer effects against various types of cancer, and the mechanism was related to inhibiting HIF-1α and VEGF expression, inhibiting the activation of PI3K/Akt and ERK1/2 pathways (32), inducing apoptosis through caspase-3, caspase-8, and caspase-9 activation and regulating Bcl-2 and Bax expression (17), and downregulating of VE-cadherin/EphA2/MMP9/MMP2 expression (15). Previous studies have shown that Rg3 has cytotoxic or cytostatic effects in lung cancer cells and in mice bearing lung cancer cells (33-35). In our present study, we extend our study to analyze the comprehensive molecular alterations of Notch signaling pathway in lung cancer cells by Ginsenoside Rg3 .Here, we showed that Ginsenoside Rg3 inhibited the growth of NCI-H1650, H520 and H1963 cells in a time and dose-dependent manner with different concentrations. Ginsenoside Rg3 induced a significant apoptosis in NCI-H1650, H520 and H1963 cells for 24 and 48 h compared to the control group. We found in the present study that the level of Notch1, Notch2 and HES1 expression decreased while the level of p38 MAPK, Caspase-3 and Caspase-9 expression increased after Ginsenoside Rg3 treatment. Hence, Ginsenoside Rg3 could target Notch/HES1 to induce the apoptotic pathway in lung cancer cell lines.
In conclusion, Ginsenoside Rg3 could target the Notch/HES1 pathway to cause apoptosis to inhibit the proliferation of lung cancer cells and the results suggested a therapeutic potential of Ginsenoside Rg3 in clinical use for lung cancer treatment.
Acknowledgments
Funding: The present study was supported by grants from the National Natural Science Foundation of China (grant No. 81303274 and 81202947) and Zhejiang Traditional Medicine Project (grant No. 2013ZA014).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.07.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jiang H, Zhao P, Feng J, et al. Effect of Paris saponin I on radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line. Oncol Lett 2014;7:2059-64. [PubMed]
- Jiang H, Zhao PJ, Su D, et al. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol Med Rep 2014;9:2265-72. [PubMed]
- Zhao P, Jiang H, Su D, et al. Inhibition of cell proliferation by mild hyperthermia at 43˚C with Paris Saponin I in the lung adenocarcinoma cell line PC-9. Mol Med Rep 2015;11:327-32. [PubMed]
- Zhang J, Yang Y, Lei L, et al. Rhizoma Paridis Saponins Induces Cell Cycle Arrest and Apoptosis in Non-Small Cell Lung Carcinoma A549 Cells. Med Sci Monit 2015;21:2535-41. [Crossref] [PubMed]
- Zhao PJ, Song SC, Du LW, et al. Paris Saponins enhance radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line by inducing apoptosis and G2/M cell cycle phase arrest. Mol Med Rep 2016;13:2878-84. [PubMed]
- Zhu X, Jiang H, Li J, et al. Anticancer Effects of Paris Saponins by Apoptosis and PI3K/AKT Pathway in Gefitinib-Resistant Non-Small Cell Lung Cancer. Med Sci Monit 2016;22:1435-41. [Crossref] [PubMed]
- Lee SY, Kim GT, Roh SH, et al. Proteomic analysis of the anti-cancer effect of 20S-ginsenoside Rg3 in human colon cancer cell lines. Biosci Biotechnol Biochem 2009;73:811-6. [Crossref] [PubMed]
- Xu TM, Cui MH, Xin Y, et al. Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J (Engl) 2008;121:1394-7. [PubMed]
- Xu TM, Xin Y, Cui MH, et al. Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide on growth and angiogenesis of ovarian cancer. Chin Med J (Engl) 2007;120:584-8. [PubMed]
- Cui W, Cheng L, Hu C, et al. Electrospun poly(L-lactide) fiber with ginsenoside rg3 for inhibiting scar hyperplasia of skin. PLoS One 2013;8:e68771 [Crossref] [PubMed]
- Yue PY, Wong DY, Wu PK, et al. The angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochem Pharmacol 2006;72:437-45. [Crossref] [PubMed]
- Lee JY, Jung KH, Morgan MJ, et al. Sensitization of TRAIL-induced cell death by 20(S)-ginsenoside Rg3 via CHOP-mediated DR5 upregulation in human hepatocellular carcinoma cells. Mol Cancer Ther 2013;12:274-85. [Crossref] [PubMed]
- Choi YJ, Lee HJ, Kang DW, et al. Ginsenoside Rg3 induces apoptosis in the U87MG human glioblastoma cell line through the MEK signaling pathway and reactive oxygen species. Oncol Rep 2013;30:1362-70. [PubMed]
- Ge X, Zhen F, Yang B, et al. Ginsenoside Rg3 enhances radiosensitization of hypoxic oesophageal cancer cell lines through vascular endothelial growth factor and hypoxia inducible factor 1α. J Int Med Res 2014;42:628-40. [Crossref] [PubMed]
- Guo JQ, Zheng QH, Chen H, et al. Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VE-cadherin/EphA2/MMP9/MMP2 expression. Int J Oncol 2014;45:1065-72. [PubMed]
- Liu T, Zhao L, Zhang Y, et al. Ginsenoside 20(S)-Rg3 targets HIF-1α to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS One 2014;9:e103887 [Crossref] [PubMed]
- Park EH, Kim YJ, Yamabe N, et al. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J Ginseng Res 2014;38:22-7. [Crossref] [PubMed]
- Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res 2009;55:1-99. [Crossref] [PubMed]
- Lee CK, Park KK, Chung AS, et al. Ginsenoside Rg3 enhances the chemosensitivity of tumors to cisplatin by reducing the basal level of nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1/NAD(P)H quinone oxidoreductase-1 and prevents normal tissue damage by scavenging cisplatin-induced intracellular reactive oxygen species. Food Chem Toxicol 2012;50:2565-74. [Crossref] [PubMed]
- Yang LQ, Wang B, Gan H, et al. Enhanced oral bioavailability and anti-tumour effect of paclitaxel by 20(s)-ginsenoside Rg3 in vivo. Biopharm Drug Dispos 2012;33:425-36. [Crossref] [PubMed]
- Kim SS, Seong S, Kim SY. Synergistic effect of ginsenoside Rg3 with verapamil on the modulation of multidrug resistance in human acute myeloid leukemia cells. Oncol Lett 2014;7:1265-69. [PubMed]
- Westhoff B, Colaluca IN, D'Ario G, et al. Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci U S A 2009;106:22293-8. [Crossref] [PubMed]
- Shih IeM, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res 2007;67:1879-82. [Crossref] [PubMed]
- Weng AP, Aster JC. Multiple niches for Notch in cancer: context is everything. Curr Opin Genet Dev 2004;14:48-54. [Crossref] [PubMed]
- Wall DS, Wallace VA. Hedgehog to Hes1: the heist of a Notch target. Cell Cycle 2009;8:1301-2. [Crossref] [PubMed]
- Bennani-Baiti IM, Aryee DN, Ban J, et al. Notch signalling is off and is uncoupled from HES1 expression in Ewing's sarcoma. J Pathol 2011;225:353-63. [Crossref] [PubMed]
- Konishi J, Kawaguchi KS, Vo H, et al. Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res 2007;67:8051-7. [Crossref] [PubMed]
- Chen Y, Li D, Liu H, et al. Notch-1 signaling facilitates survivin expression in human non-small cell lung cancer cells. Cancer Biol Ther 2011;11:14-21. [Crossref] [PubMed]
- Osanyingbemi-Obidi J, Dobromilskaya I, Illei PB, et al. Notch signaling contributes to lung cancer clonogenic capacity in vitro but may be circumvented in tumorigenesis in vivo. Mol Cancer Res 2011;9:1746-54. [Crossref] [PubMed]
- Ji X, Wang Z, Geamanu A, et al. Inhibition of cell growth and induction of apoptosis in non-small cell lung cancer cells by delta-tocotrienol is associated with notch-1 down-regulation. J Cell Biochem 2011;112:2773-83. [Crossref] [PubMed]
- Liu J, Mao Z, Huang J, et al. Blocking the NOTCH pathway can inhibit the growth of CD133-positive A549 cells and sensitize to chemotherapy. Biochem Biophys Res Commun 2014;444:670-5. [Crossref] [PubMed]
- Zeng D, Wang J, Kong P, et al. Ginsenoside Rg3 inhibits HIF-1α and VEGF expression in patient with acute leukemia via inhibiting the activation of PI3K/Akt and ERK1/2 pathways. Int J Clin Exp Pathol 2014;7:2172-8. [PubMed]
- Park D, Bae DK, Jeon JH, et al. Immunopotentiation and antitumor effects of a ginsenoside Rg3-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ Toxicol Pharmacol 2011;31:397-405. [Crossref] [PubMed]
- Kim YJ, Choi WI, Jeon BN, et al. Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-β1-induced epithelial-mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology 2014;322:23-33. [Crossref] [PubMed]
- Che JB, Liu ZH, Ma HB, et al. Influence of As2O3 combined with ginsenosides Rg3 on inhibition of lung cancer NCI-H1299 cells and on subsistence of nude mice bearing hepatoma. Asian Pac J Trop Med 2014;7:772-5. [Crossref] [PubMed]


