
Tumor-associated neutrophils: an emerging player in the immune microenvironment of hepatocellular carcinoma
Hepatocellular carcinoma (HCC), comprising the majority of primary liver cancers, is one of the leading causes of cancer-related death worldwide, responsible for approximately 746,000 cases or 9.1% of total cancer deaths in 2012 (1). HCC is commonly associated with chronic hepatitis and liver cirrhosis. Great advances have been made in the prevention, screening, and diagnosis of HCC for the past few decades. However, progress in HCC treatments has been relatively slower. Surgical resection, local ablation, and liver transplantation provide opportunities to cure early-stage diseases, but such curative treatments are associated with high recurrence rates. Sorafenib, a multikinase inhibitor mainly targeting RAF kinase and vascular endothelial growth factor receptor, is the standard therapeutic agent for advanced diseases, but shows a modest antitumor efficacy and short duration of tumor control (2,3). Thus, novel therapeutic strategies are needed (4,5).
The tumor microenvironment, particularly the immune microenvironment, has been demonstrated to mediate tumor growth, progression, metastasis, and drug resistance in various types of cancers, including HCC. An investigation of tumor-infiltrating lymphocytes showed that high Foxp3+ regulatory T cells (Tregs) and low granzyme B+ cytotoxic T cells were associated with recurrence and poor overall survival in patients with HCC after hepatectomy (6). The immunoscore, a combined analysis of CD3+ and CD8+ T cells in the tumor center and invasive margin, also predicted clinical outcome of HCC patients who had undergone hepatic resection (7). Other studies found that immune cells other than T cells, such as specific CXCR3+ B-cells, galectin-9-expressing Kupffer cells, and Tim-3-expressing tumor-associated macrophages (TAMs), also promoted HCC progression and were correlated with poor prognosis for HCC patients (8-10). Recently, Zhou et al. published an article in Gastroenterology exploring a new player, the tumor-associated neutrophils (TANs), in the pathogenesis of HCC (11). TANs were shown to recruit macrophages and Tregs to the tumor microenvironment to promote tumor growth, progression, and sorafenib resistance of HCC.
Zhou et al. first identified that CCL2 and CCL17 were the most highly expressed chemokines in TANs and peripheral blood neutrophils activated by HCC cells (11). They also demonstrated that CCL2 and CCL17 recruited CCR2+ macrophages and CCR4+ Tregs in vitro, respectively, and that TANs increased intratumoral infiltration of macrophages and Tregs in mouse liver cancer models. Co-injection of TANs with HCC cells increased tumor volume, pulmonary metastases, and neovascularization in animal models; these protumor and pro-angiogenic effects were attenuated by the depletion of TANs using an anti-Ly6G antibody or by treatment with anti-CCL2 plus anti-CCL17 antibodies. Mechanistic studies indicated that activation of p-AKT and p-P38 was involved in “educating” the peripheral blood neutrophils to become TANs by HCC cells (Figure 1A). The authors also provided clinically relevant data to support the findings of their preclinical studies, showing that CCL2 and CCL17 were expressed in the liver stroma and colocalized with the expression of a neutrophil marker (CD66b). The number of infiltrative TANs was significantly correlated with intratumoral macrophages, Tregs, and microvascular density, and the numbers of CCL2+ and CCL17+ TANs were independent poor prognostic factors in a large cohort of HCC patients who underwent curative resection.
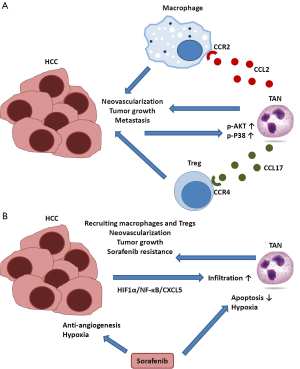
In the second part of the article, Zhou et al. explored the role of TANs in mediating the resistance to sorafenib in HCC. The authors showed sorafenib induced TAN infiltration in mouse liver cancers, and TAN infiltration was increased in HCC patients who received sorafenib treatment prior to hepatectomy (11). Mechanistically, CXCL5, which may facilitate the migration of neutrophils to tumor sites, was the strongest chemokine secreted by HCC cells under hypoxia conditions, and hypoxia-induced activation of the hypoxia-inducible factor (HIF)-1α and nuclear factor (NF)-κB pathways was the key molecular mechanism underlying CXCL5 expression in HCC cells. These mechanisms were further supported by the increased expression of HIF-1α, p65 (a subunit of NF-κB), and CXCL5 in HCC cells after sorafenib treatment in both mouse liver cancer models and HCC patients. Taken together, these data suggested that sorafenib was related to hypoxia-induced TAN infiltration via the HIF-1α/NF-κB/CXCL5 pathway (Figure 1B). Finally, the authors evaluated whether TAN-depletion therapy would enhance the therapeutic efficacy of sorafenib in mouse liver cancer models. The data showed that combination treatment with sorafenib and TAN-depletion therapy exhibited more significant inhibition of macrophage and Treg infiltration and tumor angiogenesis, and had a more profound antitumor effect than sorafenib or TAN depletion alone. In clinical samples of 46 patients with advanced recurrent HCC, patients with low TAN levels experienced significantly longer survival than those with high TANs.
The report of Zhou et al. is intriguing and provocative. But, similar to many scientific papers, their report brought up a couple of unanswered questions. The authors nicely depicted the link connecting TAN secretion of CCL2 and CCL17, intratumoral infiltration of macrophages and Tregs, and tumor progression. However, they did not determine by which mechanisms the tumor microenvironment promotes TAN infiltration. Is it mediated by the HIF-1α/NF-κB/CXCL5 pathway, which was described by the authors in the second part of the study evaluating the mechanisms of sorafenib resistance in HCC? Will blocking the CXCL5 chemokine signaling reverse the protumor and proangiogenic effects orchestrated by TANs in HCC? The authors showed HCC cells could “educate” peripheral blood neutrophils to become TANs, and the activation of PI3K/Akt and p38/MAPK signaling within neutrophils mediated the transformation of peripheral neutrophils to TANs. However, how HCC cells educate neutrophils to become TANs remains unclear. The authors also found that TANs, resulting from the activation of HIFα/NF-κB/CXCL5 by sorafenib treatment–related tumor hypoxia, may contribute to acquired sorafenib resistance in HCC. However, the clinical relevance of this proposed mechanism should be further confirmed because the clinicopathological correlation data in the report were based on only 46 advanced HCC patients and their pretreatment tissues.
Zhou et al. pointed out that TANs play a central role in the microenvironment of HCC in orchestrating the infiltration of macrophages and Tregs via CCL2-CCR2 and CCL17-CCR4 connections, respectively (11). However, the immune microenvironment of HCC and many other cancers is complex and dynamic, involving the interplay of multiple factors. For example, other studies showed that other immune cells such as specific CXCR3+ B-cells, galectin-9-expressing Kupffer cells, and Tim-3-expressing TAMs were critically important in HCC pathogenesis (8-10). Focusing on Tregs in response to sorafenib treatment in HCC, our colleagues and others found that sorafenib reduced Treg numbers or inhibited their immune suppressive functions in mouse liver cancer models or in clinical samples (12-14). On the other hand, focusing on the role of myeloid-derived suppressor cells (MDSCs), we found that sorafenib treatment induced intratumor infiltration of MDSCs and that depleting MDSCs improved the efficacy of sorafenib in orthotopic mouse liver cancer models (15). Many current experimental models, which are inevitably simple to easily demonstrate potential mechanistic insights of interest, may not truly reflect the complex and dynamic nature of the tumor immune microenvironment. Future studies should adopt more sophisticated and comprehensive approaches to better profile the immune infiltrates and their functions in the immune microenvironment of HCC.
The work of Zhou et al. has important implications in improving our current treatment of HCC patients. The importance of TANs may be incorporated into the existing data regarding the immune microenvironment of HCC. For example, how TANs are graded and added to the immunoscore and measurements of other immune cells, as well as how the quantitation of all of these immune infiltrates helps with clinical decisions in stratifying and treating HCC patients should be studied and validated in large patient cohorts. Further, strategies for depleting TANs, TAMs, or Tregs are worthy of exploration because these strategies may help delay the progression of HCC and reverse the resistance to sorafenib, as indicated by Zhou et al. An antibody targeting the CCL2-CCR2 chemokine axis was recently reported to be safe and tolerable when added to combination chemotherapy for patients with pancreatic cancer (16). An immediate translational clinical trial based on the work of Zhou et al. would be combining this antibody, which could inhibit TAMs by blocking the CCL2-CCR2 signaling axis, with sorafenib to improve the efficacy of sorafenib in advanced HCC. Well-designed clinical trials are critical for applying the findings of preclinical studies such as that of Zhou et al. to improve the treatment of HCC in the future.
Acknowledgments
Funding: This study was supported by the National Taiwan University Hospital, Taipei, Taiwan (NTUH.105-M3232).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Mu-xing Li, MD (Department of Abdominal Surgical Oncology, Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC), Beijing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.07.37). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International Agency for Research on Cancer. GLOBOCAN 2012. Available online: http://globocan.iarc.fr/
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Shao YY, Hsu CH, Cheng AL. Predictive biomarkers of antiangiogenic therapy for advanced hepatocellular carcinoma: where are we? Liver Cancer 2013;2:93-107. [Crossref] [PubMed]
- Shao YY, Hsu CH, Cheng AL. Predictive biomarkers of sorafenib efficacy in advanced hepatocellular carcinoma: Are we getting there? World J Gastroenterol 2015;21:10336-47. [Crossref] [PubMed]
- Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007;25:2586-93. [Crossref] [PubMed]
- Sun C, Xu J, Song J, et al. The predictive value of centre tumour CD8+ T cells in patients with hepatocellular carcinoma: comparison with Immunoscore. Oncotarget 2015;6:35602-15. [PubMed]
- Liu RX, Wei Y, Zeng QH, et al. Chemokine (C-X-C motif) receptor 3-positive B cells link interleukin-17 inflammation to protumorigenic macrophage polarization in human hepatocellular carcinoma. Hepatology 2015;62:1779-90. [Crossref] [PubMed]
- Li H, Wu K, Tao K, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012;56:1342-51. [Crossref] [PubMed]
- Yan W, Liu X, Ma H, et al. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut 2015;64:1593-604. [Crossref] [PubMed]
- Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016;150:1646-1658.e17. [Crossref] [PubMed]
- Chen ML, Yan BS, Lu WC, et al. Sorafenib relieves cell-intrinsic and cell-extrinsic inhibitions of effector T cells in tumor microenvironment to augment antitumor immunity. Int J Cancer 2014;134:319-31. [Crossref] [PubMed]
- Cao M, Xu Y, Youn JI, et al. Kinase inhibitor Sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab Invest 2011;91:598-608. [Crossref] [PubMed]
- Wang Q, Yu T, Yuan Y, et al. Sorafenib reduces hepatic infiltrated regulatory T cells in hepatocellular carcinoma patients by suppressing TGF-beta signal. J Surg Oncol 2013;107:422-7. [Crossref] [PubMed]
- Chang CJ, Yang YH, Chiu CJ, et al. Abstract 461: Therapeutic effect of sorafenib is attenuated by tumor-infiltrating Ly6G+ myeloid-derived suppressor cells (MDSCs) and is restored by anti-interleukin-6 (IL-6) antibody treatment in an orthotopic mouse liver cancer model. Cancer Res 2015;75:Abstract nr 461.
- Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 2016;17:651-62. [Crossref] [PubMed]


