
Urinary exosome and beyond
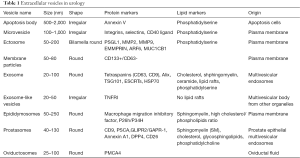
The first publication on exosomes dates back to the 1980’s. Exosomes are membrane-derived vesicles that are specified by particle size (30–100 nm in diameter), density (1.13–1.19 g/mL), and surface markers. Once referred to as a “rubbish bag” to wrap up and dump out waste, the term “exosome” gained newfound meaning following the discovery of its biogenesis mechanism via multivesicular bodies and the joint effort of Nobel Prize winners, Südhof, Schekman and Rothman, in discovering the machinery of vesicle transport. For the past decade, the number of exosome-related publications upsurged from fewer than 20 in the year 2001 to more than 1,000 in the year 2015. The nomenclature pertaining to “exosomes” was ambiguously used in literature, that is, “extracellular vesicles (EVs)” and “exosomes” were often used interchangeably or imprecisely. The criteria for exosomes have been refined over the decades to distinguish exosomes from other EVs (e.g., ectosomes, microvesicles and apoptotic bodies, see Table 1) by expression of distinct molecular markers such as TSG101, Aliex and CD63, and nanometer size in exosomes. However, the surface markers are overlapped in EV subgroups, making it fairly difficult to set a clear boundary. In this article, we will use “exosomes”, unless a specific subpopulation of EV is mentioned.
Full table
Safe and luxury journey of miRNA intercellularly
Most cells secrete exosomes, which act as an ‘intercellular postal service’ as the exosomes facilitate intercellular exchange of molecular information in the forms of protein, DNA and assorted RNA molecules. With the advantage of bilayer membrane, small fragments of RNA such as pre-miRNAs in the exosome are protected from ubiquitous RNase and undergo maturation by exosomal Dicer, Ago2 and TRBP (1). This renders the exosome a perfect shuttle of miRNA. Compared to healthy cells, cancer cells secrete a greater amount of exosomes that aid tumorigenesis. Locally, cancer exosomes not only advance cancer cells’ malignancy by promoting cell proliferation, migration, invasion and angiogenesis, but also cancerize the surrounding non-cancerous tissues. Distantly, cancer exosomes prepare an ideal metastatic site on preferential organs through circulation (2).
Obstacles of urinary exosome study
Among all body fluids, urinary exosomes provide a unique opportunity for studying urological diseases. In the May 2016 issue of the Journal of Urology, Drs. Gupta et al. published a review article on the subject of urinary exosomes for their roles in urological cancer malignancy (3). In this review, they summarize the bioactive oncoproteins and oncomiRNAs identified from urinary exosomes and derived from genitourinary cancers. The reported oncogenic properties of urinary exosomes include the promotion of cell migration and angiogenesis, aversion of apoptosis and impairing immune cells, and even facilitation of treatment resistance. Because of the huge volume and concentration variations of void urine among each individual, it is not easy to study urinary exosome on a fair platform. Identification of validated “internal” control for urine normalization is urgently needed for developing a reliable urine based bioassay. Total protein concentration, urinary creatinine levels, or particle numbers have been suggested for valuing the quantity of exosomes despite the subpopulation of exosomes that carrying different markers (4). In particular, the noticeable effect of polymeric Tamm-Horsfall protein—also known as uromodulin, and one of the most abundant proteins in urine—often diminishes the procedure’s reproducibility (5). In addition, longitudinal patients’ sample collection and verification of differences in exosome contents derived from urine as opposed to blood of the same patients will be critical information for developing the exosome-based biomarkers for monitoring tumor evolution, dynamics, and therapy response in clinical application.
Naturally-borne nanoparticles for disease biomarkers and drug delivery
More recently, scientists have also tapped into key exosome attributes for biomarkers and drug delivery potential. With minimally invasive clinical sampling and the improvement of the sensitivity of bioassays, exosome-based body fluid biopsy has opened a new avenue of biomarkers for diagnosis and prognosis of human disease. Several studies were conducted in finding miRNA, mRNA and protein content of exosomes as biomarkers such as periostin in bladder cancer (6) and PCA3 and TMPRSS2:ERG in prostate cancer (7). The updated results are summarized in Dr. Gutpa’s review. Exosomes are enriched in urine providing sources of biomarkers for urological diseases. Yet, the lack of tissue specific exosome markers that can distinguish prostate versus kidney versus bladder could hinder their further application. To determine cell origin and destination of circulating exosomes, researchers have focused on finding the specific surface molecule responsible for exosome binding and internalization of target cells. The discovery of glypican-1, a cancer exosome marker with 100% cancer specificity in pancreatic patients, generates excitement (8). It has not yet been verified if glypican-1 does present specifically in urological cancer exosomes.
As for exosomes in drug delivery application, due to their nanometer size, low immunogenicity, fast uptake rate and RNase-free environment, such naturally-borne nanoparticles become very attractive vehicles that can deliver therapeutic small molecules such as miRNA and peptides to a specific, affected area. For instance, miRNA and pharmacologic agents were reported to be successfully transferred in exosomes and delivered to cells. The exosome bearing IL-12, a key cytokine to induce tumor rejection response, has been suggested as a cancer vaccine for cancer treatment.
Something good of exosomes in urology
The prevalent body of research into exosomes has been focused on their roles in diseases, yet their roles in normal genitourinary physiology are often overlooked. For instance, in the reproductive organs, exosomes are found to be critical for gametes maturation. The ovarian follicle derived exosomes contain miRNAs that not only reflect the aging-related quality changing of oocytes, but also function in regulating estradiol and progesterone concentration levels during oocyte maturation (9,10). Epididymis and prostate secrete epididymosomes and prostasomes, enabling sperm cells to undergo necessary biochemical, biophysical, and molecular compositional changes prior to reaching oocytes to promote fertilization ability of the sperm cells (11). Furthermore, epididymosomes can transfer proteins P25b that are necessary for the sperm-egg binding, and contain the enzymes aldose reductase and sorbitol dehydrogenase that are involved in modulating sperm motility during the epididymal transition (12).
A unifying model was proposed in which prostasomes are involved in sperm capacitation and acrosome exocytosis processes (11), thereby protecting the sperm from the female’s immune system (13) and inhibiting late capacitation event and acrosome activation until sperm cells reach the oocytecumulus complex in the oviduct (14). In the kidney, exosomes are involved in cell-to-cell communication and tissue repair. Aquaporin 2 (AQP2), which functions in water molecules transfer, is one of the main components found in collecting duct cell originated urinary exosomes, and it can be delivered into AQP2 negative cells, and increase water flow (15). The TGF-β1 containing exosomes derived from injured kidney tubular epithelial cells initiate tissue regeneration (16). It is generally believed that the majority of urinary exosomes originate from renal tubular epithelia; however, their function is hardly known. Hiemstra et al. (17) conducted an in-depth research on protein contents in urinary exosomes by mass spectrometer, and surprisingly found the enrichment of antibacterial proteins and peptides. This suggested that those exosomes function as innate immune effectors for inhibiting uropathogenic bacteria in the renal tract.
Light behind the clouds
Taken together, emerging evidence has shown that urinary exosomes are involved in complex physiological and pathological genitourinary processes. This developing thought has stirred growing interest in their clinical applications. However, several issues remain critical for establishment of clinical relevance in applying exosomes. First, with respect to the mixed population of urinary exosomes, most exosome isolation methods collect particles that have the same density but not precisely separate sub-populations by their biological origin. This collection process could lead to data misinterpretation. Discovery of tissue and disease specific exosome markers would be highly significant. Second, the large variation in urine volume and density among individuals present another issue. As one example, the quantification of exosome concentration would be compromised. Identification of a stable urine normalization molecule would be critical. Lastly, the clinical studies that collect patients’ samples longitudinally with longer patient follow-up on clinical outcome are lacking. Despite these challenges, exosomes provide a novel platform for liquid biopsy for disease diagnosis, prognosis and therapy. Compared to blood borne exosome application in genitourinary diseases, urinary exosomes have the advantage of a less invasive sampling method and closer proximity to targeted secreting cells. Notwithstanding, the ability of fast uptake rate and site specific targeting makes exosomes a perfect drug delivery vehicle intravesically for bladder cancer therapy.
Acknowledgments
Funding: This work is supported by NCI R01 CA173986 (YF Lee, PI).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Peng Zhang (Department of Urology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.07.16). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Melo SA, Sugimoto H, O'Connell JT, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014;26:707-21. [Crossref] [PubMed]
- Costa-Silva B, Aiello NM, Ocean AJ, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 2015;17:816-26. [Crossref] [PubMed]
- Franzen CA, Blackwell RH, Foreman KE, et al. Urinary Exosomes: The Potential for Biomarker Utility, Intercellular Signaling and Therapeutics in Urological Malignancy. J Urol 2016;195:1331-9. [Crossref] [PubMed]
- Bobrie A, Colombo M, Krumeich S, et al. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles 2012;1. [PubMed]
- Fernández-Llama P, Khositseth S, Gonzales PA, et al. Tamm-Horsfall protein and urinary exosome isolation. Kidney Int 2010;77:736-42. [Crossref] [PubMed]
- Silvers CR, Liu YR, Wu CH, et al. Identification of extracellular vesicle-borne periostin as a feature of muscle-invasive bladder cancer. Oncotarget 2016;7:23335-45. [PubMed]
- Nilsson J, Skog J, Nordstrand A, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer 2009;100:1603-7. [Crossref] [PubMed]
- Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015;523:177-82. [Crossref] [PubMed]
- Sohel MM, Hoelker M, Noferesti SS, et al. Exosomal and Non-Exosomal Transport of Extra-Cellular microRNAs in Follicular Fluid: Implications for Bovine Oocyte Developmental Competence. PLoS One 2013;8:e78505 [Crossref] [PubMed]
- da Silveira JC, Winger QA, Bouma GJ, et al. Effects of age on follicular fluid exosomal microRNAs and granulosa cell transforming growth factor-β signalling during follicle development in the mare. Reprod Fertil Dev 2015;27:897-905. [Crossref] [PubMed]
- Yáñez-Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015;4:27066. [Crossref] [PubMed]
- Sullivan R, Saez F, Girouard J, et al. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol Dis 2005;35:1-10. [Crossref] [PubMed]
- Kelly RW. Immunosuppressive mechanisms in semen: implications for contraception. Hum Reprod 1995;10:1686-93. [PubMed]
- Ronquist G. Prostasomes are mediators of intercellular communication: from basic research to clinical implications. J Intern Med 2012;271:400-13. [Crossref] [PubMed]
- Street JM, Birkhoff W, Menzies RI, et al. Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J Physiol 2011;589:6119-27. [Crossref] [PubMed]
- Borges FT, Melo SA, Özdemir BC, et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 2013;24:385-92. [Crossref] [PubMed]
- Hiemstra TF, Charles PD, Gracia T, et al. Human urinary exosomes as innate immune effectors. J Am Soc Nephrol 2014;25:2017-27. [Crossref] [PubMed]


