
“Switchable chimeric antigen receptor T cells: a novel universal chimeric antigen receptor platform for a safe control of T-cell activation”
In a recent issue of PNAS, Rodgers et al. present an elegant study describing the development of a bifunctional antibody switch able to control retargeting and activation of chimeric antigen receptor (CAR)—engineered T cells, offering a potential additional safety tool compared to standard CAR platforms (1). Indeed, despite the recent striking responses obtained by adoptive transfer of CAR-T cells in relapsed and refractory patients affected by CD19+ malignancies, difficulties have emerged in the ability of controlling both CAR T-cell pharmacokinetic and pharmacodynamic properties (2-6).
The acute toxicities registered in the early clinical trials were strictly linked to the biological mechanism of the treatment. Currently, the observed eradication of B-cell neoplasms firstly depends on specific targeting of CD19 molecules, being highly expressed on the vast majority of leukemic clones and restricted to B-cell population. The absence of CD19 expression on other tissues granted for the safety of this approach, with the emergence of B-cell aplasia as unique “on-target” side-effect, easily treatable through immunoglobulin administration (5,6). However, the natural preference of T-cell homing to hematologic organs, i.e., the blood, bone marrow and lymph nodes allowed for rapid T-cell trafficking to tumor sites, that together with the potency of CAR-mediated antigen recognition and signal transduction, led to massive in vivo T-cell expansion, prolonged persistence and exacerbated immune responses. Especially in case of high tumor burden, serious adverse events have been observed, such as tumor lysis syndrome (TLS) and cytokine release syndrome (CRS), the latter characterized by an inflammatory systemic disease, also related to macrophage activation, mostly mediated by increased IL-6 levels (5,7). CRS have been successfully treated with the IL6R inhibitor Tocilizumab, IL-6 inhibitor Siltuximab and other anti-inflammatory drugs, such as steroids (8,9). Despite that, lack of specific control of CAR T-cell activation still remains a clinical issue that needs to be addressed.
In light of ameliorating these aspects, the work published by Rodgers and colleagues tried to address both efficacy and safety issues of CAR redirected T cells. They conceived a switchable CAR (sCAR) directed towards a peptide neo-epitope (PNE), which has been incorporated at defined different locations within an antibody targeting the CD19 antigen (antibody switch). Therefore, sCAR-T-cell specificity is redirected only against PNE, not occurring in the human proteome, thus allowing an orthogonal interaction between the sCAR-T-cell and the antibody switch. In this way, sCAR-T cells are strictly dependent on the presence of the antibody switch to become fully activated (Figure 1), thus excluding CAR T-cell off-target recognition of endogenous tissues or antigens in the absence of the antibody switch.
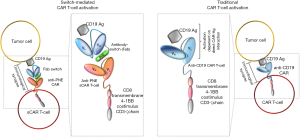
Different antibody switches were synthesized and the anti-CD19 antibody chosen was represented by clone FMC63 (10), widely used in several clinical trials, thus providing an optimal source of comparison between sCAR-T cells and traditional anti-CD19 CAR T cells (11). After the initial comparison between full antibody and single chain switches, the authors decided to only consider the Fab switch, because of a shorter pharmacokinetic half-life (~12–20 hours) (12) and smaller size, allowing for a better tumor penetration. In particular, geometry variation between the different switch molecules allowed for the dissection of a specific relationship between sCAR-T-cell functional properties and PNE location in the antibody. Indeed, superior cytotoxic activity was detected in the case of switches with PNE linked to the N terminus rather than to the C terminus, probably reflecting a proximal distance between antigen-binding interface of the antibody and target cells. The authors investigated the spacer length as another important feature of the CAR design, known to strongly affect the functional properties of CAR-redirected T cells (13,14). Moreover, a mutated version of the spacer was produced (IgG4m) in order to enhance interchain sCAR disulfide formation, allowing for dimerization of sCAR, leading to increased T-cell activation. This strategy showed to be beneficial, with shorter and IgG4m spacers providing increased cytotoxic activity and also improved sCAR-T-cell activation.
Finally, the authors demonstrated the efficacy of this approach in vivo, with an increasing dose of antibody switch. However, at the ideal switch concentration no amelioration of the signs related to both CRS and TLS were observed in comparison to the anti-CD19 CAR T-cell strategy, with a daily or every other day administration. Therefore, the authors suggested that, in order to grant the control over the disease, the ideal solution should be that of titrating the switch dose, starting with a low switch dose administration, able to mitigate both CRS and TLS, in high tumor burden settings. In this way, it could be possible to control sCAR-T-cell activation without switching off the system, as it happens when dealing with suicide gene strategies (15), that usually lead to a complete abrogation of the therapeutic efficacy. Ideally, the control of sCAR-T-cell activity would be enough to dampen the manifestation of the toxicity associated with CRS, but not excessive to cause disease relapse. In this sense, the limited half-life of the Fab molecule could be beneficial, given the immediate result over sCAR-T-cell activation when turning off the administration of the switch. However, the relapse risk upon excessive control over sCAR-T cells remains the crucial question mark, despite successive reinfusion of the switch might theoretically result in the recovery of sCAR-T-cell effector functions. Conversely, when dealing with lower tumor burden (i.e., MRD state), an increased switch dose concentration could be offered, without the risk of exacerbating CRS and TLS events. Besides, the described strategy offers an alternative to the use of a suicide gene in case of long-lasting T-cell populations, with the aim to revert the observed persistent B cell aplasia.
The perspectives of the transition from an antibody switch to another represent a valid tool in case of antigen escape or of cancer with a heterogeneous antigen expression. Specifically, therapies targeting CD19 on leukemic blasts, such as anti-CD19 CAR T cells or the bi-specific anti-CD19/CD3 antibodies blinatumomab experienced CD19-negative relapses, with alternatively spliced CD19 isoforms, reported in approximately 10% of treated patients. Novel CAR T-cell approaches are under evaluation to target additional antigens, such as TSLPR and CD22, for the treatment of relapses with target epitope loss (16,17). Future directions could include a preemptive combination of more antibody switches or bi-specific switches in order to avoid emergence of antigen loss escape mutations.
Concerning the control of potential “on-target but off tumor” recognition of healthy cells, studying the best Fab switch affinity could be an aspect worth to be further deepened, in order to limit potential acute side effects, particularly in the context of TAA that once targeted are potentially life-threatening. Indeed, tuning the affinity of antigen binding domain of the CAR turned out to strongly affect the functional properties of the redirected CAR T cells in an extremely context dependent manner (18-20). In this way, rationale administration of low doses switch with a limited affinity for the target antigen could offer a safer strategy also for the treatment of both solid tumors and other hematological malignancies.
Overall, this approach presents the great promise of combining both the titrability of antibody-based therapies with the efficacy of redirected CAR T cells. The clinical translation of the proposed approach is very interesting, particularly concerning the possibility of developing a universal sCAR-T-cell, obviating thus the need of creating a new CAR for each target. However, for each platform proposed this strategy demands for the optimization of the exact immunological synapse orientation in order to elicit the best sCAR-T-cell effector functions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xia Fang (Department of hematology, Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.07.23). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rodgers DT, Mazagova M, Hampton EN, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci U S A 2016;113:E459-68. [Crossref] [PubMed]
- Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38 [PubMed]
- Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540-9. [Crossref] [PubMed]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517-28. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139 [Crossref] [PubMed]
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188-95. [Crossref] [PubMed]
- Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127:3321-30. [Crossref] [PubMed]
- Teachey DT, Lacey SF, Shaw PA, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov 2016;6:664-79. [Crossref] [PubMed]
- Nicholson IC, Lenton KA, Little DJ, et al. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol Immunol 1997;34:1157-65. [Crossref] [PubMed]
- Bridgeman JS, Hawkins RE, Hombach AA, et al. Building better chimeric antigen receptors for adoptive T cell therapy. Curr Gene Ther 2010;10:77-90. [Crossref] [PubMed]
- Flanagan RJ, Jones AL. Fab antibody fragments: some applications in clinical toxicology. Drug Saf 2004;27:1115-33. [Crossref] [PubMed]
- Hombach AA, Schildgen V, Heuser C, et al. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J Immunol 2007;178:4650-7. [Crossref] [PubMed]
- Guest RD, Hawkins RE, Kirillova N, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother 2005;28:203-11. [Crossref] [PubMed]
- Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011;365:1673-83. [Crossref] [PubMed]
- Qin H, Cho M, Haso W, et al. Eradication of B-ALL using chimeric antigen receptor-expressing T cells targeting the TSLPR oncoprotein. Blood 2015;126:629-39. [Crossref] [PubMed]
- Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 2013;121:1165-74. [Crossref] [PubMed]
- Hudecek M, Lupo-Stanghellini MT, Kosasih PL, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res 2013;19:3153-64. [Crossref] [PubMed]
- Chmielewski M, Hombach A, Heuser C, et al. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol 2004;173:7647-53. [Crossref] [PubMed]
- Arcangeli S, Bardelli M, Tettamanti S, et al. Unraveling the efficacy and safety profiles of anti-CD123 chimeric antigen receptors (CARs) in a model of acute myeloid leukemia monotherapy by investigating CAR binding affinity and density variables. Blood 2015;126:1359.


