
Long-term use of sunitinib in metastatic renal cell carcinoma: no unpleasant surprises about tolerability
Metastatic relapse occurs in approximately 30% of patients who have previously undergone nephrectomy for renal cell carcinoma (1). Anti-angiogenic agents, such as tyrosine kinase inhibitors (TKI), have played a leading role in the treatment of metastatic renal cell carcinoma (mRCC) because it is highly resistant to chemotherapy. Sunitinib is a first-line TKI targeting both the vascular endothelial growth factor receptor (VEGFR) and the platelet-derived growth factor receptor (PDGFR). Treatment of mRCC with sunitinib has been shown to increase progression-free survival (PFS) to 11 months (95% confidence interval 0.32–0.54) versus 5 months with baseline interferon alpha treatment (P<0.001) (1), and to improve overall survival (OS) and quality of life. However, patients receiving sunitinib experience specific treatment-related adverse events, including hypertension, epistaxis, hypothyroidism, hand-foot syndrome, hair color changes, fatigue, diarrhea and hematological toxicity. Although almost all patients will develop secondary resistance to sunitinib, 10% remain progression free during >24 months’ therapy (2). This chronic use of sunitinib raises the question of long-term safety for patients treated for more than 2 years (3).
A recent study by Porta et al. provided long-term follow-up data on more than 5,000 patients from phase II and III clinical trials who received sunitinib for mRCC, 1% of whom were treated for at least 5 years (4). The results showed that grade 3 and 4 treatment-related adverse events reached a peak during the first year of treatment, while rates of most adverse events were stable over the duration of treatment. Specifically, hand-foot syndrome, which is an important cause of treatment discontinuation due to its negative impact on quality of life, occurred at a constant rate of about 30%. The rate of hypertension was also stable around 30% for the 6 years of treatment. This finding contradicts the results of other studies that have reported large, treatment duration-related increases in systolic and diastolic blood pressure during sunitinib treatment (5).
The mechanism by which TKIs cause hypertension has been suggested to be a inhibition of VEGFR-2 signalling and reduced nitric oxide production, resulting in systemic vasoconstriction (6). It is possible that the finding of a stable long-term hypertension rate by Porta et al. (4) may be the result of antihypertensive treatment given to control blood pressure while on sunitinib. The study does not report data on concomitant medication or the type of antihypertensive agents used, some of which might have increased activity due to interaction with sunitinib (7). Hypothyroidism appeared to be the only cumulative toxicity of sunitinib, and is related to vascular regression of capillaries in the thyroid and induction of type 3 deiodinase activity, as well as a reduction in triiodothyronine (T3) and thyroxine (T4) levels (8,9).
The study by Porta et al. was the first to gather long-term safety data on sunitinib. The results are quite reassuring given that they don’t show cumulative toxicity after more than 2 years’ sunitinib treatment, except for hypothyroidism that can be managed with hormonal substitution. However, there is a lack of information regarding the sunitinib dose given, and the dose of sunitinib in current practice tends to reduce over time. It is also important to take into account the sunitinib administration schedule (2/1 versus 4/2), which was not reported but could reduce toxicity (10).
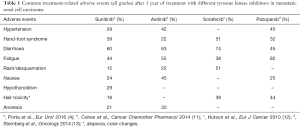
The success of TKIs for increasing survival in mRCC means that a number of additional agents have been developed and are in use as first-line or second-line treatment (Table 1). However, long-term safety remains an issue and there are fewer data on these newer agents. Over a median treatment duration of nearly 3 years with axitinib, the most common grade 3/4 treatment-related adverse events (including hypertension, diarrhea, proteinuria and fatigue) occurred in the first 6 months (11). In another analysis of pooled data of long-term axitinib use, rates of proteinuria, peripheral edema and renal failure increased over time, whereas the rate of all other adverse events remained stable or decreased (14). In the TARGET study, Hutson et al. (12) reported moderate toxicities after 1-year of treatment with sorafenib, such as diarrhea, cutaneous adverse events (rash or desquamation), hand-foot syndrome, alopecia, and fatigue, most of which appeared early during treatment. Similar findings have been reported for pazopanib (15). In one study, over a median 9.7 months’ treatment, recurrent adverse events were hypertension, diarrhea, hair color changes, anorexia and nausea (13); in this study toxicity was lower than that reported in the COMPARZ trial (16). Overall, current data suggest that all available TKIs share the same safety profile.
Full table
The next question is whether new targeted therapies for mRCC will challenge existing TKIs in term of efficacy and safety. Cabozantinib is a new TKI targeting MET, VEGFR, and AXL. In the METEOR trial, second-line treatment of mRCC with cabozantinib was associated with better PFS and OS than mTOR inhibitors (17). With a median follow-up of 18.8 months, the most frequent treatment-related adverse events were similar to those with currently-available TKIs, and the main grade 3/4 events were hypertension, diarrhea, fatigue, hand-foot syndrome, anemia, hyperglycemia and hypomagnesemia (17); there were no data regarding toxicities over time. Immunotherapy has shown promise in the treatment of mRCC resistant to anti-angiogenic therapies. The recent CheckMate 025 study brought hope for second-line treatment of mRCC with nivolumab, an anti-PD1 agent, which was shown to be more effective than everolimus (18). After treatment for a median of more than 8 months, OS was of 28.1 months, although the rate of any grade treatment-related adverse events and immune-mediated toxicities was 70% (18).
Available data suggest that the long-term safety of TKIs has been well assessed, and it is now widely accepted that the most severe treatment-related adverse events occur in the first year of therapy. However, some toxicities remain difficult to manage on a long-term basis (e.g., hypertension, hypothyroidism and hand-foot syndrome). Education of both patients and clinicians is important to decrease the intensity of side effects and a lot of work has been done in this regard. New therapies are challenging TKIs for first- and second-line treatment of mRCC. However, there are few data on long-term safety compared with well-known TKIs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hong-Chao He, MD, PhD (Department of Urology, Shanghai Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.07.48). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24. [Crossref] [PubMed]
- Tannir NM, Figlin RA, Gore ME, et al. Long-term response to sunitinib treatment in metastatic renal cell carcinoma (mRCC). J Clin Oncol 2016;34:abstr 598. Available online: http://meetinglibrary.asco.org/content/158387-172
- Molina AM, Jia X, Feldman DR, et al. Long-term response to sunitinib therapy for metastatic renal cell carcinoma. Clin Genitourin Cancer 2013;11:297-302. [Crossref] [PubMed]
- Porta C, Gore ME, Rini BI, et al. Long-term Safety of Sunitinib in Metastatic Renal Cell Carcinoma. Eur Urol 2016;69:345-51. [Crossref] [PubMed]
- Azizi M, Chedid A, Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med 2008;358:95-7. [Crossref] [PubMed]
- Robinson ES, Khankin EV, Karumanchi SA, et al. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol 2010;30:591-601. [Crossref] [PubMed]
- McKay RR, Rodriguez GE, Lin X, et al. Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Cancer Res 2015;21:2471-9. [Crossref] [PubMed]
- Makita N, Miyakawa M, Fujita T, et al. Sunitinib induces hypothyroidism with a markedly reduced vascularity. Thyroid 2010;20:323-6. [Crossref] [PubMed]
- Kappers MH, van Esch JH, Smedts FM, et al. Sunitinib-induced hypothyroidism is due to induction of type 3 deiodinase activity and thyroidal capillary regression. J Clin Endocrinol Metab 2011;96:3087-94. [Crossref] [PubMed]
- Bracarda S, Iacovelli R, Boni L, et al. Sunitinib administered on 2/1 schedule in patients with metastatic renal cell carcinoma: the RAINBOW analysis. Ann Oncol 2016;27:366. [Crossref] [PubMed]
- Cohen EE, Tortorici M, Kim S, et al. A Phase II trial of axitinib in patients with various histologic subtypes of advanced thyroid cancer: long-term outcomes and pharmacokinetic/pharmacodynamic analyses. Cancer Chemother Pharmacol 2014;74:1261-70. [Crossref] [PubMed]
- Hutson TE, Bellmunt J, Porta C, et al. Long-term safety of sorafenib in advanced renal cell carcinoma: follow-up of patients from phase III TARGET. Eur J Cancer 2010;46:2432-40. [Crossref] [PubMed]
- Sternberg CN, Davis ID, Deen KC, et al. An open-label extension study to evaluate safety and efficacy of pazopanib in patients with advanced renal cell carcinoma. Oncology 2014;87:342-50. [Crossref] [PubMed]
- Rini BI, Escudier B, Hariharan S, et al. Long-Term Safety With Axitinib in Previously Treated Patients With Metastatic Renal Cell Carcinoma. Clin Genitourin Cancer 2015;13:540-7.e1-7.
- Zivi A, Cerbone L, Recine F, et al. Safety and tolerability of pazopanib in the treatment of renal cell carcinoma. Expert Opin Drug Saf 2012;11:851-9. [Crossref] [PubMed]
- Choueiri TK, Figueroa DJ, Fay AP, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res 2015;21:1071-7. [Crossref] [PubMed]
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17:917-27. [Crossref] [PubMed]
- Escudier BJ, Motzer RJ, Sharma P, et al. Treatment beyond progression with nivolumab (nivo) in patients (pts) with advanced renal cell carcinoma (aRCC) in the phase III CheckMate 025 study. J Clin Oncol 2016;34:abstr 4509.


