
Hepatitis B virus core-related antigen is a serum prediction marker for hepatocellular carcinoma
Introduction
Serum hepatitis B virus (HBV)-DNA is a useful measurement for chronic hepatitis B (CH-B) patients and can help determine when to start antiviral therapies, such as nucleos(t)ide analogues (NAs) and interferon (IFN), as well as monitoring of the antiviral effects of therapy. It is reported that the development of advanced chronic liver disease, cirrhosis, and the incidence of hepatocellular carcinoma (HCC) can be prevented by decreasing serum HBV-DNA levels. Serum HBV-related markers can be conveniently measured along with other serum liver function markers such as AST, ALT, rGTP, and ALP at regular intervals from small serum samples. However, serum HBV-DNA levels in many cases during NAs treatment are below detection limits despite the presence of HBV-related proteins in patients’ hepatocytes. HBV-DNA decrease is not the aim of therapy in recent years; rather, the absence of HBs antigen has become essential. This indicates that HBs antigen loss is associated with intrahepatic HBV loss, so a HBV marker that reflects the production of intrahepatic HBV is needed. Kimura et al. reported that HBV core-related antigen (HBcrAg) reflects HBcAg in Dane particles, p22cr in empty particles, and HBeAg, and is detected by CLEIA method after pretreatment to inactivate the antibodies (1). The results of the study suggested that most Dane particles lack viral HBV-DNA and core capsids, but contain p22cr (2). This study additionally provided a model for the formation of the HBV-DNA-negative Dane particles. The precore proteins, which lack the arginine-rich nucleotide-binding domain, form viral RNA/DNA-negative capsid-like particles and are enveloped and released as empty particles. To measure HBcrAg, antibodies such as HBeAb and HBcAb are inactivated and HBV core antigen (HBcAg) is contained within the Dane particles. However, HBcAg cannot be directly detected in serum, unlike HBcAb, which indicates only past and current infection status. The lower limit of detection of HBcrAg is 3.0 logU/mL and the upper limit is 7.0 logU/mL, and detection reflects the production of intrahepatic HBV including HBV cccDNA, which if eradicated, corresponds with decreased HBcrAg in serum. HBcrAg includes HBeAg but is of little use in HBeAg-positive patients. On the other hand, measurement is useful when serum HBV-DNA cannot be detected and the patient has a negative HBeAg and positive HBeAb status. Previous reports showed that there is a significant correlation between intrahepatic HBV cccDNA and the detection of serum HBcrAg (3,4). Moreover, HBcAg staining in liver tissue has been found to be associated with serum HBcrAg (2).
HBcrAg is a useful serum marker for estimating the intrahepatic replication of HBV, and if it cannot be detected during NAs therapy, HBV is considered to be completely suppressed, meaning that therapy can be discontinued. For example, this may occur when HBV-DNA is suppressed and the patient’s transaminase levels remain within the normal range after several months of NAs treatment. Conversely, if the value is above the normal range for HBcrAg, it shows that HBV-related proteins are being produced and NAs treatment should not be discontinued because hepatitis would recur. Some reports have shown that discontinuation of NAs does not lead to reactivation in cases of low HBcrAg (5,6). In these patients, HBV cccDNA may be at a low level and unable to produce HBV-related antigens including HBcAg in Dane particles. In fact, very little HBV cccDNA can be detected in liver tissue in these cases, and it is undetectable by RTD-PCR-based assays after the isolation of genomic DNA (3). Rokuhara et al. compared levels of HBcrAg with HBV-DNA levels during lamivudine treatment and found higher viremia in HBcrAg-positive patients. The authors concluded that HBcrAg assay is a sensitive and useful test for assessing HBV load (7). Shinkai et al. reported that HBcrAg is useful for predicting relapses after discontinuation of lamivudine treatment (6). They found that HBcrAg levels of <3.4 logU/mL when lamivudine was discontinued represented the only independent factor that predicted the absence of post-treatment relapse. Furthermore, Matsumoto et al. reported in 2007 that HBcrAg could be a useful marker for identifying patients who are not at risk of reactivation of severe hepatitis after discontinuation of lamivudine (5). The authors found that HBcrAg serum levels were significantly higher (P<0.01) in patients who experienced reactivation, and lower levels indicated a lower risk. Matsuzaki et al. reported that HBcrAg is a useful HBV re-infection marker after liver transplantation (4). Moreover, they highlighted that maintaining negative levels of HBcrAg and HBV cccDNA might contribute to long-term graft survival. Seto et al. analyzed and characterized both linearized hepatitis B surface antigen (HQ-HBsAg) and HBcrAg during the natural history of CH-B (8). They showed that the measurement of these markers is crucial and they can be detected even in cases of HBsAg sero-clearance. Maasoumy et al. suggested that HBcrAg might be an additional marker of HBV infection (9). The group performed a study of a European cohort in HBeAg-negative patients predominantly infected with genotypes A and B, and concluded that HBcrAg might help to distinguish between inactive carriers and cases of active disease. In 2015, Matsumoto et al. conducted a retrospective study of factors associated with the patient outcome of IFN sequential therapy, which was established so that NAs could be discontinued (10). The authors concluded that the combined detection of HBsAg and HBcrAg levels might be useful for predicting the 24-month outcome of sequential therapy. Chuaypen et al. compared HBcrAg against quantitative HBsAg in patients with HBeAg-positive CH-B receiving PEG-IFN therapy (11). They concluded that HBcrAg levels measured during PEG-IFN therapy might help identify patients with a very low probability of response, which is comparable with, if not better than, quantitative HBsAg. van Campenhout et al. measured HBcrAg among patients treated with NAs with or without PEG-IFN add-on therapy (12). The result showed that the decline in HBcrAg was stronger in patients given combined therapy than in those given NAs alone. The authors also mentioned that HBcrAg was associated with a combined response but was not superior to quantitative HBsAg. Zhang et al. compared HBcrAg values and pathological status using clinical liver tissues (13). They concluded that HBcrAg could predict severe necroinflammation and advanced fibrosis in HBeAg-positive patients, as well as significant necroinflammation and fibrosis in HBe-negative patients.
HBcrAg measurement is reported to be useful for predicting HCC incidence in CH-B patients (Table 1). The reports show a direct or indirect association between HBcrAg and HCC incidence, whether under NAs treatment, or via observation of the natural course of the disease. Hosaka et al. first reported the association of HBcrAg with HCC and analyzed the risk factors recurrent during NAs treatment (14). An analysis of intrahepatic HBV cccDNA showed that high levels were associated with HCC incidence, and the authors concluded that HBcrAg was a risk indicator of HCC recurrence. Akuta et al. investigated the relationship between HCC and HBsAg (15). The authors compared HBsAg and AFP and found an association between low HBsAg and low HBcrAg; in these cases, HCC did not develop. The group measured AFP at the start of their observations and reported that initial high levels (>10 μg/L) are associated with HCC incidence (P<0.001).
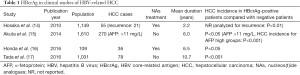
Full table
We introduced the significance of measuring HBcrAg and related it to intra-hepatic HBV replication and the development of HCC (16). We could not find any significant association between HBcrAg values and cancer incidence before NAs treatment. On the other hand, we observed a greater incidence of HCC in HBcrAg-positive cases compared with negative cases during NAs treatment, and HCC incidence was associated with liver fibrosis status using the FIB-4 index. The incidence of HCC cannot be accurately assessed if patients are still HBeAg-positive after NAs administration and if minor seroconversion has not taken place. However, many of our cases exhibited minor seroconversion, and we were able to compare HCC and non-HCC cases with HBeAg-negative status. Using microarray analysis, we found that transcriptional factors including HNF4α, PPARα, and LRH1 are upregulated in HBcrAg-positive livers. The overexpression of HBV precore/core in HepG2 increased in vitro, but these transcription factors were reduced by Metformin in HBV-infected primary hepatocytes.
In an analysis of CH-B patients not treated with antivirals, Tada et al. reported that the development of HCC is associated with the elevation of HBcrAg levels (17). They found that the elevation of both HBcrAg and basic core promoter (BCP) mutation were independently associated with the incidence of HCC. Moreover, time-dependent ROC analysis showed that HBcrAg is superior to HBV-DNA in terms of its prediction of the development of HCC during the follow-up period. This data indicated that HBcrAg is better at predicting HCC incidence at all times compared with serum HBV-DNA. These results suggest that HBcrAg is closely associated with the production of intrahepatic HBV proteins.
Tada et al. collected information on 3,122 HBV-positive patients and excluded patients under treatment before conducting a final analysis on 1,031 patients that fulfilled the criteria. The subjects were 473 female and 558 male patients. During the follow-up period, 162 developed cirrhosis and 78 developed HCC. The cumulative HCC incidence was 2.0% for 5 years, 8.3% for 10 years, 10.7% for 15 years, and 12.5% for 20 years. In addition, they conducted multivariate analysis and found that HBcrAg (HR 5.05; 95% CI, 42.40–10.63; P<0.001) and BCP mutation (HR 28.85; 95% CI, 4.00–208.20; P<0.001) were associated with the incidence of HCC. They then analyzed the cumulative incidence of HCC combined with HBcrAg and BCP status and found that patients with HBcrAg <2.9 logU/mL and BCP wild status represented 0% of the incident rate of HCC even up to 20 years later. Moreover, HBcrAg >2.9 logU/mL and BCP mutant status was 6.4% for 5 years, 25.0% for 10 years, 30.1% for 15 years, and 30.4% for 20 years, suggesting that positivity for both represents a definite risk for HCC. They also analyzed HBV-DNA and found that values of >5 log copies/mL were significantly associated with the incidence of HCC; however, values of <5 log copies/mL showed no significant correlation. Multivariate analysis of HBV genotypes, HBV-DNA, HBcrAg (cut off >2.9 logU/mL), HBeAg, and BCP mutation, showed that HBcrAg of >2.9 logU/mL and BCP mutation were associated with the risk of HCC incidence. In an analysis of HCC incidence among patient subgroups of those with low HBsAg levels (<3 logU/mL) and HBV genotype C, it was found that in both subgroups, HBcrAg levels of >2.9 logU/mL were significantly associated with the incidence of HCC. They characterized a subgroup that had HBV-DNA levels <4.0 log copies/mL, and were HBeAg negative and non-cirrhotic (n=581). Among this subgroup, 17 patients developed HCC and had HBcrAg levels of >3.7 logU/mL, but not HBcrAg >2.9 logU/mL or HBsAg >3 logU/mL, showing that high HBcrAg levels were significantly associated with the incidence of HCC. On the other hand, among 376 patients with HBV-DNA >4 log copies/mL, any HBeAg status, and an FIB-4 index of <3.6, 36 developed HCC and there was significant association with HBcrAg >2.9 and >3.7 logU/mL groups. A time-dependent ROC curve illustrated the levels of HBcrAg and HBV-DNA alongside the incidence of HCC at 2, 3, 4, 5, 6, 7, 8, 9, and 10 years, and they found that the AUCs were greater for HBcrAg than HBV-DNA at all points in time, suggesting that HBcrAg provides a superior estimation of HCC incidence than HBV-DNA. However, one limitation of the study was that it was conducted within a single hospital-based population and they suggested that data should be collected from community-based populations. Another limitation was that none of the CH-B patients received NAs, which means that this study involved a low-risk cohort. They concluded that HBcrAg levels are associated with the development of HCC in CH-B patients not receiving antiviral therapy.
More than 10 years have passed since the introduction of HBcrAg measurement in Japan, and its value in patients under NA treatment via the analysis of many HBV-infected patients has been reported. The most useful purpose of these evaluations has been to help clarify when to discontinue NAs in inactive hepatitis patients. However, the advantage of this marker is diminished in cases of persistent HBeAg positivity, because the assay detects HBeAg in addition to HBcAg. This marker is associated with HCC incidence, especially in cases of HBeAg-negative and HBeAb-positive patients. As this paper has reported, even in non-treated cases, HBcrAg is more useful for estimating cancer risk than other markers, such as HBV-DNA using ROC analysis.
Conclusions
Serum HBcrAg reflects intrahepatic HBV production more accurately than HBV-DNA. This marker is associated with HCC incidence, and is applicable to NAs or IFN non-treated patients. Tada et al. measured and compared serum HBcrAg and HBV-DNA annually in follow-up using a ROC curve, and discovered that the AUC value of HBcrAg was superior compared with serum HBV-DNA. Although it is a serum assay, several reports have shown that the measurement of HBcrAg is advantageous in the evaluation of intrahepatic HBV.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Mu-Xing Li, MD (Department of Abdominal Surgical Oncology, Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC), Beijing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.08.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kimura T, Rokuhara A, Sakamoto Y, et al. Sensitive enzyme immunoassay for hepatitis B virus core-related antigens and their correlation to virus load. J Clin Microbiol 2002;40:439-45. [Crossref] [PubMed]
- Kimura T, Ohno N, Terada N, et al. Hepatitis B virus DNA-negative dane particles lack core protein but contain a 22-kDa precore protein without C-terminal arginine-rich domain. J Biol Chem 2005;280:21713-9. [Crossref] [PubMed]
- Suzuki F, Miyakoshi H, Kobayashi M, et al. Correlation between serum hepatitis B virus core-related antigen and intrahepatic covalently closed circular DNA in chronic hepatitis B patients. J Med Virol 2009;81:27-33. [Crossref] [PubMed]
- Matsuzaki T, Tatsuki I, Otani M, et al. Significance of hepatitis B virus core-related antigen and covalently closed circular DNA levels as markers of hepatitis B virus re-infection after liver transplantation. J Gastroenterol Hepatol 2013;28:1217-22. [Crossref] [PubMed]
- Matsumoto A, Tanaka E, Minami M, et al. Low serum level of hepatitis B core-related antigen indicates unlikely reactivation of hepatitis after cessation of lamivudine therapy. Hepatol Res 2007;37:661-6. [Crossref] [PubMed]
- Shinkai N, Tanaka Y, Orito E, et al. Measurement of hepatitis B virus core-related antigen as predicting factor for relapse after cessation of lamivudine therapy for chronic hepatitis B virus infection. Hepatol Res 2006;36:272-6. [Crossref] [PubMed]
- Rokuhara A, Tanaka E, Matsumoto A, et al. Clinical evaluation of a new enzyme immunoassay for hepatitis B virus core-related antigen; a marker distinct from viral DNA for monitoring lamivudine treatment. J Viral Hepat 2003;10:324-30. [Crossref] [PubMed]
- Seto WK, Wong DK, Fung J, et al. Linearized hepatitis B surface antigen and hepatitis B core-related antigen in the natural history of chronic hepatitis B. Clin Microbiol Infect 2014;20:1173-80. [Crossref] [PubMed]
- Maasoumy B, Wiegand SB, Jaroszewicz J, et al. Hepatitis B core-related antigen (HBcrAg) levels in the natural history of hepatitis B virus infection in a large European cohort predominantly infected with genotypes A and D. Clin Microbiol Infect 2015;21:606.e1-10. [Crossref] [PubMed]
- Matsumoto A, Yatsuhashi H, Nagaoka S, et al. Factors associated with the effect of interferon-α sequential therapy in order to discontinue nucleoside/nucleotide analog treatment in patients with chronic hepatitis B. Hepatol Res 2015;45:1195-202. [Crossref] [PubMed]
- Chuaypen N, Posuwan N, Payungporn S, et al. Serum hepatitis B core-related antigen as a treatment predictor of pegylated interferon in patients with HBeAg-positive chronic hepatitis B. Liver Int 2016;36:827-36. [Crossref] [PubMed]
- van Campenhout MJ, Brouwer WP, van Oord GW, et al. Hepatitis B core-related antigen levels are associated with response to entecavir and peginterferon add-on therapy in hepatitis B e antigen-positive chronic hepatitis B patients. Clin Microbiol Infect 2016;22:571.e5-9. [Crossref] [PubMed]
- Zhang ZQ, Lu W, Wang YB, et al. Measurement of the hepatitis B core-related antigen is valuable for predicting the pathological status of liver tissues in chronic hepatitis B patients. J Virol Methods 2016;235:92-8. [Crossref] [PubMed]
- Hosaka T, Suzuki F, Kobayashi M, et al. HBcrAg is a predictor of post-treatment recurrence of hepatocellular carcinoma during antiviral therapy. Liver Int 2010;30:1461-70. [Crossref] [PubMed]
- Akuta N, Suzuki F, Kobayashi M, et al. Correlation between hepatitis B virus surface antigen level and alpha-fetoprotein in patients free of hepatocellular carcinoma or severe hepatitis. J Med Virol 2014;86:131-8. [Crossref] [PubMed]
- Honda M, Shirasaki T, Terashima T, et al. Hepatitis B Virus (HBV) Core-Related Antigen During Nucleos(t)ide Analog Therapy Is Related to Intra-hepatic HBV Replication and Development of Hepatocellular Carcinoma. J Infect Dis 2016;213:1096-106. [Crossref] [PubMed]
- Tada T, Kumada T, Toyoda H, et al. HBcrAg predicts hepatocellular carcinoma development: An analysis using time-dependent receiver operating characteristics. J Hepatol 2016;65:48-56. [Crossref] [PubMed]


