
Elucidating alternative pathways triggering small cell lung carcinoma tumor biology
In their recent perspective Ito et al. comprehensively reviewed the carcinogenesis of pure and combined small cell lung carcinoma (SCLC) (1) and we appreciate the explicit discussion of our article Meder et al. “NOTCH, ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas.” (2).
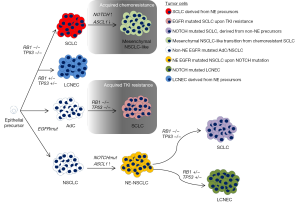
Ito et al. augmented the regulators of neuroendocrine (NE) differentiation. In addition to achaete-scute homolog 1 (ASCL1), they highlighted Brain-2 (BRN2) and insulinoma-associated protein 1 (INSM1) upstream of ASCL1 which regulate NE marker expression and differentiation in normal pulmonary epithelial cells and cancer cells (1). It remains to be explored, whether BRN2 and INSM1 indeed autonomously drive a morphological switch from non-small cell lung cancer (NSCLC) towards a SCLC phenotype as it has been shown for ASCL1 (2,3). However, all three factors comprise a NE signaling network regulated by NOTCH-Hes1 signaling (1).
Critically, inactivating mutations in NOTCH genes seemed to be not sufficient but advantageous for SCLC formation. In our study, we found evidence for genetic inactivation of NOTCH receptors driving NOTCH-ASCL1 dependent NE differentiation in NE-NSCLC, large cell NE carcinomas (LCNEC) and the so called secondary SCLC transitions from NSCLC (2). However, Ito et al. summarized also the findings of Niederst et al. who reported that bi-allelic loss in RB1 alone was responsible for the transition from EGFR mutated adenocarcinomas (AdC) to SCLC. Furthermore, their results from whole exome sequencing did not reveal genetic alterations in NOTCH genes (4). As the NOTCH loci harbor extremely GC-rich sequences, they are frequently under-covered by next generation sequencing analyses (5) and hence, inactivating mutations in NOTCH genes are frequently unreported.
However, in a Cre inducible SCLC mouse model using cell type specific Adeno-Cre, non-NE pulmonary cells such as alveolar type 2 cells served as origin for SCLC upon full RB and p53 inactivation, without any additional genetic NOTCH depletion (6).
Importantly, Ito et al. pointed out, that it remains elusive how RB loss triggers NE differentiation especially with regard to SCLC transition from AdC with acquired therapy resistance (1).
In addition, the question was raised whether combined SCLC may differentiate from pure SCLC upon deregulation of NOTCH signaling and reduction of ASCL1 expression. Brambilla et al. showed already in 1991 in patients suffering from chemoresistant SCLC that tumor cells acquired a more differentiated phenotype and an increased cell size upon therapy resistance (7). Calbó et al. showed in 2005, that there was tumor heterogeneity within SCLC lesions comprising NE and non-NE tumor cells. Here, they proposed for the non-NE tumor cells an important role in acquiring chemoresistance (8). Interestingly, in 2011 they were able to link oncogenic Ras protein expression to a switch from NE to a mesenchymal non-NE phenotype (9).
Consequently, comprehensive signaling pathway analysis is required to elucidate how NOTCH, RB and Ras may cooperate in the induction of SCLC and SCLC-NSCLC transitions (Figure 1) and how they can mediate therapy resistance. For this purpose, robust and deep analysis of clinical cases is needed to finally overcome acquired therapy resistance and to improve patient outcome. This is a compulsive issue especially for highly aggressive neoplasms, such as SCLC. Thus, it is essential to routinely isolate biopsies from patients before, during and after therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.08.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ito T, Matsuo A, Hassan WA. Notch signaling and Tp53/RB1 pathway in pulmonary neuroendocrine tumorigenesis. Transl Cancer Res 2016;5:213-9. [Crossref]
- Meder L, König K, Ozretić L, et al. NOTCH, ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. Int J Cancer 2016;138:927-38. [Crossref] [PubMed]
- Osada H, Tomida S, Yatabe Y, et al. Roles of achaete-scute homologue 1 in DKK1 and E-cadherin repression and neuroendocrine differentiation in lung cancer. Cancer Res 2008;68:1647-55. [Crossref] [PubMed]
- Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015;6:6377. [Crossref] [PubMed]
- Alioto TS, Buchhalter I, Derdak S, et al. A comprehensive assessment of somatic mutation detection in cancer using whole-genome sequencing. Nat Commun 2015;6:10001. [Crossref] [PubMed]
- Sutherland KD, Proost N, Brouns I, et al. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011;19:754-64. [Crossref] [PubMed]
- Brambilla E, Moro D, Gazzeri S, et al. Cytotoxic chemotherapy induces cell differentiation in small-cell lung carcinoma. J Clin Oncol 1991;9:50-61. [PubMed]
- Calbó J, Meuwissen R, van Montfort E, et al. Genotype-phenotype relationships in a mouse model for human small-cell lung cancer. Cold Spring Harb Symp Quant Biol 2005;70:225-32. [Crossref] [PubMed]
- Calbo J, van Montfort E, Proost N, et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 2011;19:244-56. [Crossref] [PubMed]


