
Tailor the adjuvant hormonal manipulation for premenopausal breast cancer patients
The 2016 publication titled “The hormonal manipulation 5-Fluoro-uracil Epirubicin Cyclophosphamide (HMFEC) trial” in the European Journal of Cancer provided an opportunity to revisit the adjuvant HM of premenopausal breast cancer (BC) patients (1). In the HMFEC study, premenopausal BC patients with positive lymph node involvement were randomized in a 2×2 factorial fashion to groups either administered FE50C vs. FE75C followed by HM or no HM. Gonadotrophin-releasing hormone agonists (GnRHa) were used for HM if patients retained menstrual cycle after chemotherapy; for patients became amenorrhea after chemotherapy, tamoxifen was administered. As expected, HM benefit was not seen in ER-negative population. In the ER-positive/ER-unknown subpopulation, the HM provided modest and nonsignificant recurrence reduction [hazard ratio (HR) 0.85, 95% confidence interval (CI) 0.62–1.17, P=0.32] (1). The authors justified the unexpected results in ER-positive premenopausal BC patients by citing inadequate power and small event number. In addition, the HM used in this trial is generally considered an inadequate adjuvant endocrine therapy for premenopausal BC patients. Nevertheless, HMFEC was designed 30 years ago to tackle an important question of whether HM is beneficial for premenopausal BC patients who had received chemotherapy, at a time that the 1998 Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis has shown that adding either tamoxifen or ovarian ablation to chemotherapy in premenopausal patient did not improve patient outcome (2). Thirty-years later, with mounting evidences, the 2016 American Society of Clinical Oncology (ASCO) guideline now suggests that tamoxifen +/− ovarian suppression, or ovarian suppression + aromatase inhibitor (AI) to be a treatment of choice for premenopausal BC patients (3). The findings of the HMFEC trial and the paradigm shift in the guideline from 1998 to 2016 provide an opportunity to examine the history and optimization of the adjuvant endocrine treatment for ER-positive premenopausal BC patients.
Efficacy of adjuvant ovarian ablation or suppression
In the 1996 EBCTCG meta-analysis, ovarian ablation with oophorectomy, radiation, or GnRHa was shown to reduce BC recurrence and death in patients aged younger than 50 years, but only reached statistical significance in the chemotherapy-absent subgroup (4). In the follow-up 2005 EBCTCG meta-analysis, it revealed similar but significant risk reduction of BC recurrence and mortality by ovarian ablation or suppression in patients under the age of 50, but with a more modest effect for those who had received chemotherapy as part of the adjuvant treatment (5). In contrast, in the 2007 meta-analysis by the LHRH-agonists in Early Breast Cancer Overview Group, the addition of a LHRH agonist with or without tamoxifen after chemotherapy significantly reduced the risk of recurrence or death, especially in patients under the age of 40, a group who were less likely to become amenorrheic after chemotherapy (6). This was the first signal suggesting that addition of ovarian suppression maybe beneficial for those who remained premenopausal after adjuvant chemotherapy. In the Suppression of Ovarian Function Trial (SOFT), ovarian suppression plus tamoxifen did not show significant improvement in disease-free survival (DFS) and overall survival (OS) as compared with tamoxifen only in the overall study population of premenopausal BC patients; however, when the analysis was limited to those who had a higher risk of recurrence thus to receive either neoadjuvant or adjuvant chemotherapy, the effect on DFS and OS was more pronounced and significant (Table 1) (7). Based on the updated results, current ASCO guideline recommends tamoxifen plus or minus ovarian suppression as the choice of adjuvant hormonal therapy for premenopausal BC patients.
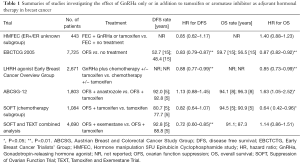
Full table
Comparison between tamoxifen and AI with the use of ovarian ablation or suppression: conflicting data
For postmenopausal BC patients, AI is generally considered more advantageous than tamoxifen in terms of both BC control and OS (8). Whether this statement holds true in the premenopausal setting remains to be determined. In the phase III neoadjuvant clinical trial (STAGE) comparing goserelin plus either anastrozole or tamoxifen in premenopausal BC women in Japan, the anastrozole arm had a significantly higher tumor response rate as compared with tamoxifen (70.4% vs. 50.5%, P=0.004), suggesting a higher anti-tumor efficacy of AI as compared with tamoxifen in premenopausal patients receiving GnRHa (9). However, in Austrian Breast and Colorectal Cancer Study Group 12 (ABCSG12) study, where premenopausal women were randomized to either goserelin plus anastrozole or goserelin plus tamoxifen, there was no significant difference in terms of DFS at the first 5 years (HR 1.10; 95% CI, 0.78–1.53; P=0.59) (10). After a median of 8 years of follow-up, the final analysis of OS showed that the anastrozole arm had a significant worse survival as compared with the tamoxifen arm (HR 1.63; 95% CI, 1.0–1.45; P=0.03) (11). There are various explanations for this unexpected result (discussed later) but this also further complicates the choice of hormonal agent along with GnRHa as adjuvant hormonal therapy for premenopausal women. The recent joint analysis of the SOFT and Tamoxifen and Exemestane Trial (TEXT) demonstrated different results as compared to ABCSG12. Ovarian suppression plus exemestane had a significant DFS benefit (HR 0.72; 95% CI, 0.60–0.85; P<0.001) as compared with ovarian suppression plus tamoxifen. Other endpoints such as rate of freedom from BC or distant metastasis showed similar trend, but the OS were not different between the two arms (HR 1.14; 95% CI, 0.86–1.51; P=0.37) (12). The benefit of exemestane on DFS was also more profound in those who received chemotherapy. Based on these results, current ASCO guideline suggests that for premenopausal BC patients with a higher risk of recurrence, a GnRHa should be added to either tamoxifen or AI (3).
Reasons for the conflicting results of STAGE, ABCSG12 trial and SOFT/TEXT joint analysis
Two major reasons have been proposed to explain the poor survival of the anastrozole arm in the ABCSG12 trial. First, a high body mass index (BMI) may reduce the efficacy of AI. Patients with a higher BMI tend to have a higher proportion of fat, which leads to increased aromatase production; therefore, the flat dose of anastrozole may have been insufficient to achieve complete estrogen suppression. The secondary analysis of ABCSG12 showed that patients who were overweight (BMI ≥25 kg/m2) had a nearly 50% increase in the risk of disease recurrence (HR 1.49; 95% CI, 0.93–2.38; P=0.08) and a three-fold increase in the risk of death (HR 3.03; 95% CI, 1.35–6.82; P=0.004) compared with tamoxifen-treated patients (13). The BMI of Asian patients is generally lower than that of Caucasian patients; this may partly explain the conflicting results of the neoadjuvant STAGE and adjuvant ABCSG12 trials.
Second, AI could stimulate ovarian estrogen production through the rebound of follicle-stimulating hormone (FSH) by stimulating the hypothalamus-pituitary pathway (14). Dowsett et al. recently cautioned that the plasma estradiol (E2) levels can increase in some individuals after the first month of GnRHa plus exemestane, though the mean level of E2 is lower than that after GnRHa plus tamoxifen administration (15). In the SOFT trial, 25% of the patients were found to have incomplete estrogen suppression [plasma E2 level ≥2.72 pg/mL detected by gas chromatography tandem mass spectrometry (GC/MS/MS) assay] at least once in the first year of triptorelin plus exemestane treatment. Baseline factors associated with an on-treatment E2 level ≥2.72 pg/mL (10 pmol/L) included no prior chemotherapy, higher BMI, and lower FSH and luteinizing hormone (LH) level (16). The proportion of patients receiving chemotherapy may also explain the conflicting results of the clinical trials. Adjuvant chemotherapy was not allowed in the ABCSG12 and only 5–6% of the patients received neoadjuvant chemotherapy (10). By contrast, 57.4% of the patients in the SOFT/TEXT joint analysis received chemotherapy (12). Although all patients in SOFT/TEXT remained premenopausal at the time entering the study, chemotherapy has been shown to induce premature ovarian failure, which decreases the risk of incomplete ovarian suppression (17).
However, despite evidences suggested that AI may be superior in terms of BC recurrence control as compared with tamoxifen in premenopausal BC patients receiving GnRHa, AI was not superior to tamoxifen in terms of OS in both ABCSG12 and the SOFT/TEXT combined analysis (11,12). The morbidities associated with long-term metabolic and osteoporosis side effects of AIs should be taken into account when determine the choice of hormonal agent for premenopausal BC patients receiving GnRHa (18).
Current recommendations for tailoring adjuvant endocrine therapy for premenopausal BC patients
To optimize the adjuvant endocrine therapy, physicians must estimate its absolute benefit of recurrence and mortality reduction. The ASCO 2016 guideline recommends that higher-risk patients should receive ovarian suppression in addition to adjuvant endocrine therapy, whereas lower-risk patients should not; ovarian suppression may be administered with either tamoxifen or AI (3).
Although the clinical significance of incomplete ovarian suppression by GnRHa remains uncertain, many researchers have suggested that regular biochemical testing of estrogen should be seriously considered for premenopausal patients receiving GnRHa in addition to an AI as an adjuvant endocrine treatment (15,19). For example, Papakonstantinou et al. proposed an algorithm recommending GnRHa plus AI for 5 years in high-risk patients and/or aged ≤35 years who have received chemotherapy, and monitor the E2, FSH, and LH levels every 3–6 months during the period. If adequate ovarian suppression cannot be achieved, alternative treatment possibilities including switching to tamoxifen plus ovarian suppression with GnRHa for at least 5 years or bilateral oophorectomy plus AI should be sought (19).
In the real-world practice, plasma E2 level may not be measured through GC/MS/MS assay in most hospitals. For patients who did not receive adjuvant chemotherapy, the use of tamoxifen with or without GnRHa is recommended. For patients who received adjuvant chemotherapy, ovarian suppression is recommended, but the choices between tamoxifen and AI remains controversial. Although AI seems to be more efficacious in preventing BC recurrence, this is counterbalanced by the lack of differences in OS between GnRHa plus AI group vs. GnRHa plus tamoxifen group and the trade-off of possibly increased risk of adverse events of AI and incomplete ovarian suppression. Physicians should thoroughly discuss the pros and cons of adjuvant HM to premenopausal BC patients to reach a tailored therapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor San-Gang Wu (Department of Radiation Oncology, Xiamen Cancer Center, the First affiliated Hospital of Xiamen University, Xiamen, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.08.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coombes RC, Kilburn LS, Tubiana-Mathieu N, et al. Epirubicin dose and sequential hormonal therapy-Mature results of the HMFEC randomised phase III trial in premenopausal patients with node positive early breast cancer. Eur J Cancer 2016;60:146-53. [Crossref] [PubMed]
- . Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. Early Breast Cancer Trialists' Collaborative Group. N Engl J Med 1988;319:1681-92. [Crossref] [PubMed]
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol 2016;34:1689-701. [Crossref] [PubMed]
- . Ovarian ablation in early breast cancer: overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet 1996;348:1189-96. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [Crossref] [PubMed]
- LHRH-agonists in Early Breast Cancer Overview group. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet 2007;369:1711-23. [Crossref] [PubMed]
- Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 2015;372:436-46. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341-52. [Crossref] [PubMed]
- Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol 2012;13:345-52. [Crossref] [PubMed]
- Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 2009;360:679-91. [Crossref] [PubMed]
- Gnant M, Mlineritsch B, Stoeger H, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol 2015;26:313-20. [Crossref] [PubMed]
- Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014;371:107-18. [Crossref] [PubMed]
- Pfeiler G, Königsberg R, Fesl C, Mlineritsch B, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol 2011;29:2653-9. [Crossref] [PubMed]
- Casper RF, Mitwally MF. Review: aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab 2006;91:760-71. [Crossref] [PubMed]
- Dowsett M, Lønning PE, Davidson NE. Incomplete Estrogen Suppression With Gonadotropin-Releasing Hormone Agonists May Reduce Clinical Efficacy in Premenopausal Women With Early Breast Cancer. J Clin Oncol 2016;34:1580-3. [Crossref] [PubMed]
- Bellet M, Gray KP, Francis PA, et al. Twelve-Month Estrogen Levels in Premenopausal Women With Hormone Receptor-Positive Breast Cancer Receiving Adjuvant Triptorelin Plus Exemestane or Tamoxifen in the Suppression of Ovarian Function Trial (SOFT): The SOFT-EST Substudy. J Clin Oncol 2016;34:1584-93. [Crossref] [PubMed]
- Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol 2008;26:753-8. [Crossref] [PubMed]
- Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst 2011;103:1299-309. [Crossref] [PubMed]
- Papakonstantinou A, Foukakis T, Rodriguez-Wallberg KA, et al. Is Estradiol Monitoring Necessary in Women Receiving Ovarian Suppression for Breast Cancer? J Clin Oncol 2016;34:1573-9. [Crossref] [PubMed]


