
Durvalumab (MEDI4736; anti-PD-L1 inhibitor): to shed light on the treatment of advanced urothelial bladder cancer patients refractory to systemic chemotherapy
For several decades, chemotherapy regimens for advanced urothelial cancer of the urinary bladder have not changed, and they include methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) and gemcitabine and cisplatin (GC) with similar efficacy and relatively less toxicity (1). Moreover, there are a few second-line chemotherapeutic agents demonstrating substantial therapeutic responses in patients who are resistant to platinum-based treatments (2). Although the novel targeted agents may offer alternative therapeutic options, their efficacies are still limited and further investigations are needed (3). Recently, many researchers have shown great interest in the role of local and systemic immune responses, which interplay with cancer cells, as a novel therapeutic target (4).
In this regard, the identification of immune checkpoint molecules and a better understanding of the mechanisms of a complex interplay between tumor and immune cells has promoted the modulation of host immune responses in cancer patients (5-7). Particularly, the breakthrough in developing monoclonal antibodies to specifically block the immune checkpoint proteins, such as programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1), has revolutionized the conventional therapeutic strategy in patients with advanced stage cancer, including urothelial bladder cancer (UBC) (8-12).
Massard et al. (13) recently published promising results in the Journal of Clinical Oncology. This study was an international, phase 1/2 open label, dose-escalation and expansion study, which included a total of 61 patients with advanced UBC treated by the anti-PD-L1 inhibitor durvalumab (MEDI4736, 10 mg/kg every 2 weeks) via intravenous infusion for up to 12 months. The primary end point of this study was safety on the basis of assessment of adverse events (AEs) and serious AEs. Safety was assessed from the initiation of study through 90 days after the last day of durvalumab treatment. Secondary end points were objective response rate (ORR), defined as a confirmed complete or partial response, and disease control rate (DCR) at 12 weeks, defined as a confirmed complete or partial response, or stable disease for ≥12 weeks. They reported that thirty-nine patients (63.9%) showed a drug-related AE of any grade as the primary end point. Fatigue (13.1%), diarrhea (9.8%), and decreased appetite (8.2%) were reported as the most frequent drug-related AEs. Of note, grade 3 AEs only occurred in three patients (4.9%), and grade 4 to 5 AEs were not observed during the study periods. The ORR was 31% in 42 evaluable patients [95% confidence interval (CI), 17.6–47.1], and the median duration of response was 6.3 months (95% CI, 5.6–12.1).
Another key finding was that 40 patients (66.6%) were PD-L1 positive and 21 patients (34.4%) were PD-L1 negative on immunohistochemical (IHC) staining of biopsy specimens. More importantly, the ORR of durvalumab in advanced UBC patients was 31%, particularly 46.4% (95% CI, 27.5–66.1) in the PD-L1 positive subgroup versus 0% (95% CI, 0.0–23.2) in the PD-L1 negative population. Similar to these findings, Herbst and colleagues found that patients with high PD-L1 expressing tumors were significantly responsive to atezolizumab in various type of malignancies, such as non-small cell lung cancer, renal cell carcinoma, melanoma and colorectal cancer (14). The authors speculated that atezolizumab can be most effective in cancer patients in whom the host immune status is attenuated by PD-L1, and therefore, it can be boosted by treatment with a PD-L1 inhibitor. Taken together, the study by Massard et al. first reported that durvalumab showed tolerable safety results as well as substantial clinical effectiveness in advanced UBC patients, particularly the PD-L1 positive subgroup.
To better understand the results and clinical significance of this article, the recent progress and molecular mechanisms of anti-PD-L1 immune check point inhibitors are reviewed. We further discuss how it became a promising agent in patients with advanced stage cancer, including UBC, particularly, those patients who had been heavily pretreated.
Mechanistically, malignant cells highly express the immune checkpoint molecule PD-L1, as the ligand for immunoreceptor PD-1 on lymphocytes in order to evade the cytotoxic effects of host immune cells (5). It results in inhibition of T-cell-mediated anti-cancer activities by negatively regulating the intracellular signaling pathway of T-cell activation (5). In this regard, researchers have successfully discovered that specific inhibitors of immune checkpoint molecules could enhance the anti-cancer effects of the host immune system. PD-1 and PD-L1 inhibitors specifically bind to PD-1 on immune cells and PD-L1 on cancer cells, respectively (15,16). Thus, they finally block the interaction between PD-1 on immune cells and PD-L1 on cancer cells, and enhance the T-cell-mediated anti-cancer activities (15,16).
In 2011, the US Food and Drug Administration (FDA) approved ipilimumab (CTLA4 inhibitor) as a therapeutic agent for patients with metastatic melanoma, which was the first landmark in the practical use of immune checkpoint inhibitors (17). The US FDA also approved two PD-1 inhibitors (nivolumab and pembrolizumab) for patients with metastatic melanoma (18,19). Particularly, nivolumab showed meaningful activity in other types of advanced solid tumors, such as squamous non-small cell lung cancer, renal cell carcinoma and colorectal cancer (8). Pembrolizumab also demonstrated significant efficacy in patients with triple-negative breast cancer, non-small cell lung cancer, and advanced gastric cancer (20).
Similar to the promising results of PD-1/PD-L1 inhibitors for patients with various malignancies, some individuals with advanced UBC also showed remarkable responses to these immune checkpoint inhibitors (21). Actually, previous studies revealed that PD-L1 expression was related to pathologic and oncologic outcomes in urothelial cancer. Inman et al. (22) examined the expression levels of PD-L1 in surgical specimens of 280 UBC patients, and found that higher expression of PD-L1 was significantly associated with high-grade and advanced-stage tumors. Moreover, the study by Boorjian et al. (23) showed that the PD-L1 expression in cystectomy specimens was an independent predictive factor for overall survival following surgery.
On the basis of these findings, Powles et al. (12) first performed a phase I trial in 68 patients with metastatic UBC who were treated with the anti-PD-L1 antibody MPDL3280A (atezolizumab; Tecentriq®). The authors observed that the objective response rate was 43% in the subpopulation expressing higher PD-L1 expression compared to the objective response rate of 11% in patients showing low PD-L1 expression in specimens, and they published these promising results in Nature (12). From these data, the US FDA finally approved this drug for advanced UBC in June 2014. Rosenberg and colleagues also published promising results of a single-arm, multicenter, phase 2 trial of atezolizumab in 310 patients with locally advanced or metastatic UBC (24). The objective response rate was 26% in patients with higher PD-L1 expression on tumor-infiltrating lymphocytes, whereas the overall population showed an objective response rate of 15%. More importantly, they first revealed that there was a significant association between responses to PD-L1 blockade and TCGA subtypes, suggesting the effect of the genomic landscape in advanced bladder cancer on the responsiveness to immune checkpoint inhibitors. Following the success of atezolizumab in bladder cancer, other promising PD-1/PD-L1 inhibitors are currently undergoing clinical trials (Table 1).
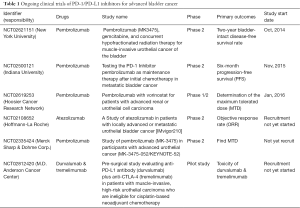
Full table
Despite the recently published positive data, the discrepancy regarding the criteria for PD-1/PD-L1 expression in tumor cells or tumor infiltrating immune cells between studies is an unavoidable limitation. Comparative analyses between various methods for PD-1/PD-L1 staining in specimens are required to identify the reason for discrepant outcomes among studies. Moreover, further study with a large study population should be performed to validate the previous findings based on a different assay, such as SP263 used in this phase 2 study of durvalumab. Finally, the consensus on the criteria for PD-1/PD-L1 expression in tumor microenvironment can offer valuable clinical information that will help select patients for treatment with PD-1 or PD-L1 inhibitors from among advanced UBC patients who are refractory to systemic chemotherapy.
In conclusion, the study by Massard et al. provides the promising evidence that blocking PD-1 and PD-L1 is an efficacious way to treat advanced stage bladder cancer patients who have been previously treated with systemic chemotherapy. Furthermore, it is crucial to identify the specific populations of patients who may be benefited more by the use of anti-PD1 or PD-L1 inhibitors, such as durvalumab, compared to other treatments. We believe that these novel agents will become the standard therapy for unhopeful UBC patients who fail to respond to cisplatin-based chemotherapy, and finally, the first-line treatment would be changed from cisplatin-based chemotherapy to immune checkpoint inhibitors for advanced UBC in the near future.
Acknowledgments
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2016R1A2B4011623).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hong-Chao He MD, PhD (Department of Urology, Shanghai Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sternberg CN, Bellmunt J, Sonpavde G, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur Urol 2013;63:58-66. [Crossref] [PubMed]
- Ortmann CA, Mazhar D. Second-line systemic therapy for metastatic urothelial carcinoma of the bladder. Future Oncol 2013;9:1637-51. [Crossref] [PubMed]
- Fletcher A, Choudhury A, Alam N. Metastatic bladder cancer: a review of current management. ISRN Urol 2011;2011:545241.
- Pennock GK, Chow LQ. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist 2015;20:812-22. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discov 2014;13:883-4. [Crossref] [PubMed]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974-82. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [Crossref] [PubMed]
- Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 2014;15:69-77. [Crossref] [PubMed]
- Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol 2015;33:1430-7. [Crossref] [PubMed]
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-62. [Crossref] [PubMed]
- Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Zhan MM, Hu XQ, Liu XX, et al. From monoclonal antibodies to small molecules: the development of inhibitors targeting the PD-1/PD-L1 pathway. Drug Discov Today 2016;21:1027-36. [Crossref] [PubMed]
- Li Y, Li F, Jiang F, et al. A Mini-Review for Cancer Immunotherapy: Molecular Understanding of PD-1/PD-L1 Pathway & Translational Blockade of Immune Checkpoints. Int J Mol Sci 2016;17. [PubMed]
- Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res 2012;18:2039-47. [Crossref] [PubMed]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Khoja L, Butler MO, Kang SP, et al. Pembrolizumab. J Immunother Cancer 2015;3:36. [Crossref] [PubMed]
- Carosella ED, Ploussard G, LeMaoult J, et al. A Systematic Review of Immunotherapy in Urologic Cancer: Evolving Roles for Targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol 2015;68:267-79. [Crossref] [PubMed]
- Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer 2007;109:1499-505. [Crossref] [PubMed]
- Boorjian SA, Sheinin Y, Crispen PL, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res 2008;14:4800-8. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]


