
Feed your gut with caution!
Introduction
Mammalian intestine is lined by a single layer of epithelia, serving as the first cell type to come into contact with food-derived antigens, toxins, bile acids, digestive enzymes, and the vast number microorganisms collectively known as the gut microbiota. To withstand constant exposure to environmental and physiological stressors, the gut epithelium organized its architecture into specialized protrusions and crevices. As per the dictum ‘form follows function’, the intestinal villi are constituted with enterocytes and goblet cells to facilitate digestion, nutrient absorption and mucin secretion for lubrication. Colonic crypts, on the other end, are invaginations, which also house the delicate stem cells at the base, presumably for seclusion from the adverse gut environments (Figure 1). Yet, the exact compounds, microbes or their metabolites that need to be kept away from the colonic crypts remain to be characterized.
From numbers to noxious metabolites
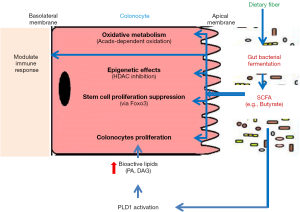
Our understanding of the gut microbiota has rapidly expanded over the recent decades, in part, due to the advent of next-generation sequencing and multi-omics technologies. In due course, the microbiota research productively transitioned from asking “what microbes are there?” to inquiring “what are the microbes doing?”, especially in regards to their influences on gut physiology and the overall host metabolism. In a study published in Cell, Kaiko et al. (1) employed the Cdc25A promoter-driven luciferase reporter assay as an innovative high throughput approach to screen for microbial metabolites that may impact the proliferation of intestinal stem/progenitor cells. Out of the 92 metabolites screened, Kaiko et al. identifies eight candidate microbial metabolites—m-toluic acid, 4-hydroxyindole, indole 3-acetamide, tyramine, pyridoxal, spermidine, deoxycholic acid and butyrate—that could endanger the crypt-residing stem cells. Their findings now illustrate that butyrate, derived from bacterial fermentation of dietary fibers, is perhaps the most harmful and potent at suppressing the proliferation of stem/progenitor cells in the crypts.
Dietary fibers (DF, a complex carbohydrate) are the indigestible portion of plant foods primarily from fruits, vegetables and grains. Although mammalian digestive tract can’t metabolize DF, soluble fibers such as inulin and its oligofructose derivatives are readily fermented by the gut microbiota, generating large quantities of short-chain fatty acids (SCFAs; e.g., acetate, butyrate and propionate) in ~ concentration of 80–130 mM (2,3). Butyrate, the four-carbon SCFA, displays the most diverse and seemingly contradictory effects on cell proliferation, differentiation and apoptosis which may exert both pro- or anti-tumorigenic effects, a phenomenon termed as butyrate paradox. In addition, its biological effects may be also influenced by factors such as site, level of exposure, availability of other metabolic substrates and intracellular milieu.
Stappenbeck’s group postulate that the inhibitory effect of butyrate on colonic stem cells could be mitigated by their strategic placement in crypts, thus limiting their exposure to the metabolite. To gain mechanistic insights, the authors examined the zebrafish, a model organism known to lack colonic crypts (4). Intriguingly, they discovered that both butyrate and butyrate-producing microbes are conspicuously absent in the zebrafish crypt-less gut. When Kaiko et al. treated zebrafish with butyrate, the proliferation of colonic epithelial cells in zebrafish was significantly suppressed thus reaffirming the authors’ hypothesis. These exciting findings not only support role of crypt in preventing butyrate from reaching the stem cells within, but also paint a new picture on the evolutionary basis for the development of crypts in animals that harbor butyrate-producing microbes.
Location, location and location
The concentration of luminal butyrate tends to be highest in the cecum, the ‘anaerobic bioreactor’ where maximal microbial fermentation of DF occurs, but progressively decreases at locations further away. SCFAs are notably minimal in the small bowel due to absence of butyrate producing microbes. However, the colonic landscape is completely reversed in the large bowel which is devoid of extended villi in addition to harboring increased load of microbes and their metabolites.
Crypts in the small bowel may be accommodated with reduced levels of SCFA, but how would the large bowel safeguards its crypts from butyrate? Fascinatingly, Kaiko et al. reveal that colonocytes as the ‘crypt-guards’ are equipped with a metabolic machinery to rapidly utilize and deplete butyrate, thus providing an additional layer of protection. Using colonic administration of 13C-labeled butyrate, the authors demonstrate that most of the butyrate is maximally absorbed by the colonocytes at top of the crypt. They further confirmed that in mice whose colonocytes could not metabolize butyrate due to genetic loss of acyl-CoA dehydrogenase (Acads; a key enzyme that converts butyrate to acetyl-CoA), the proliferative capacity of crypt stem cells were indeed attenuated, presumably inhibited by butyrate.
To depend or dispense of butyrate
The major butyrate producers identified are mostly from the phyla Firmicutes (e.g., Clostridium spp., Roseburia spp., Eubacterium spp., Faecalibacterium prausnitzii) that expresses the butyryl-CoA transferase. Yet, their absence in zebrafish implicates that butyrate-producers in general are dispensable for zebrafish, even though their gut displayed high epithelial turnover rates. On the contrary, the notion that butyrate is an essential and major (constituted >70%) energy source for mammalian colonic epithelial cells is well-accepted (5). It is rather unusual that colonic epithelia in mammals have to depend on a microbial product when the organism can adequately support many large organs independently of butyrate. The study by Kaiko et al. supports the prospect that butyrate utilization by colonocytes may not necessarily reflect a simple nutritional requirement, but rather as a metabolic sinkhole. By electing to utilize butyrate, the colonocytes may have gained two potential advantages: (I) deplete butyrate thus preventing it from flooding the crypts; and (II) avoid from exhausting the more precious energy commodity (i.e., glucose). These hypotheses, however, may need to be further verified using germ-free mice. Regardless of whether it is by chance or design, the utilization of butyrate by the colonocytes undoubtedly presents an intriguing example of how the host and gut microbiota co-evolved into a state of coexistence.
Even so, it may be crucial to revisit the rationale behind linking a decrease in certain bacteria groups with pathological conditions. Many studies reported the tremendous reduction in major butyrate-producers in the gut of individuals with inflammatory bowel diseases (IBD) (6-9). While the loss of butyrate-producers is hallmarks of gut dysbiosis, it remains unclear whether such alterations are cause or consequence of the disease, or perhaps a natural phenomenon that require no further intervention. Many would consider such microbial changes to be detrimental and would advocate pro- and pre-biotic approaches as potential treatments. However, it is not a foregone conclusion that the loss of butyrate producers may actually be beneficial for the diseased host, whose gut may not be in the best condition to tolerate luminal butyrate.
Fueling the gut with butyrate: beneficial or inflammatory hazard
To examine the effects of butyrate during IBD, Kaiko et al. administered mice with dextran sodium sulfate (DSS; a chemical colitogen) to induce colonic ulceration, thus exposing the stem cells to luminal butyrate. The suppression of stem cell activity can be detrimental during colitis where high turnover of colonic epithelial cells is required to seal and heal the disrupted colonic epithelium in response to DSS-induced injury. Indeed, the proliferation of stem cells in DSS-treated mice was suppressed, which is accompanied by aggravated ulceration and delayed wound repair. Fascinatingly, the administration of metronidazole to selectively deplete butyrate-producing bacteria (BPB) ameliorated DSS-induced colonic ulceration, whereas exogenous butyrate or fecal transplant with BPB re-ignited the colitis phenotype. These observations not only illustrate the adverse effects of butyrate on gut health, but also allude to its potentially detrimental effect to individuals with IBD.
Similar to the study by the Stappenbeck group, Zhang et al. also examined the unfavorable effect of BPB in mice with DSS-induced colitis (10). The administration of human-derived BPB strain Anaerostipes hadrus was demonstrated to elevate the levels of luminal butyrate in healthy mice, but not in colitic mice. The DSS-treated mice that received A. hadrus displayed an exacerbated colitis instead (10), thus implicating that the effects of butyrate (as well as producers and fiber precursors) may critically depend on the gut physiology. This notion is further supported by another study in which the supplementation of soluble fiber inulin resulted in increased colonic expression of inflammatory genes in IBD-prone interleukin (IL)-10 deficient mice, but not in wild-type mice (11). It is possible that gut microbiota-derived metabolites including butyrate might contribute to colonic inflammation or potentially explain why mice with gut dysbiosis are sensitive to develop colitis (12-14); however, this conjecture warrants further pondering.
Collectively, these studies challenge our pre-existing notion that metabolites produced by microbial fermentation of dietary fiber are ‘beneficial’ for gut health in all conditions including IBD. As shown by Kaiko et al., the healthy colonocytes are equipped with the metabolic enzymes of TCA cycle and lipid metabolism, and also possess a high ratio of oxygen consumption rate (an indicator of oxidative phosphorylation) to extracellular acidification rate (an indicator of glycolysis). This allows the colonocytes to rapidly oxidize butyrate, thus instituting a metabolic barrier to protect stem/progenitor cells. However, during IBD where numbers of colonocytes are substantially reduced, the microbial metabolites such as butyrate may worsen the pathology by delaying the epithelial repair and wound-healing. These assertions coincide with the current practices to exclude fermentable oligosaccharides, disaccharides, monosaccharides and polyols (aka FODMAPs) from the diet of irritable bowel syndrome patients (15). Therefore, it is perhaps not surprising that a subset of IBD patients exhibit IBD flares upon consuming a fiber rich diet. Indeed, patients with IBD often develop intestinal side effects to DF at doses well-tolerated by >90% of healthy subjects (16). It does not seem that much of a stretch to contemplate that SCFAs may be beneficial when it is well-tolerated by a normal gut, but became detrimental when in excess or not handled well by a dysfunctional gut.
Decrypting the butyrate paradox
The contention whether SCFAs and its soluble fiber precursors are beneficial or detrimental has been the subject of much debate. Such controversy can be traced as far back to the 1980s, at a time not long after butyrate was established to be a potent inhibitor of histone deacetylase (HDAC) (17,18). As studies began to explore the epigenetic-modulating properties of butyrate, it was discovered that butyrate can exert opposing effects on healthy and cancerous colonic cells in regards to their proliferation, differentiation and apoptosis (19). Even after decades of ensuing research, the ‘butyrate paradox’ is far from being resolved but instead became further convoluted with reports on the conflicting intra- and extra-intestinal effects of butyrate, SCFAs and DF. Notably, previous studies have demonstrated the beneficial properties of SCFAs on suppressing colonic inflammation (20-24), improving satiety (25), and reducing hepatic lipogenesis and adiposity (26-28). Yet on the flip side, the more studies began to unravel that inulin (29) as well as intrarectal butyrate (30) fuel the transformation of colonic epithelia and causes colorectal cancer (29), SCFAs aggravate colonic inflammation (10,31), induce urethritis and hydronephrosis (32), and promote obesity by aggravating hepatic lipogenesis (33) and hyperphagia (34).
In addition to epigenetic effects, another potential bioactivity of butyrate is promoting cell proliferation. Butyrate is known to upregulate phospholipase D1 (PLD1) in gut epithelia (35). PLD1 hydrolyzes the phosphodiester bond of phosphatidylcholine (PC), a major phospholipid in cell membranes, generating phosphatidic acid (PA) and choline implicated in membrane trafficking/membrane fusion (36). Further, PA can be converted into bioactive lipids, including diacylglycerol (DAG), lysophosphatidic acid (LPA) and cyclic phosphatidic acid (CPA) (37). These bioactive lipids are potent second messengers/mitogens and promote cell proliferation, migration and invasion.
The study by Kaiko et al. provides yet another key evidence on the potency of butyrate as an HDAC inhibitor (38) and a potentially harmful epigenetic-modulating metabolite. In line with previous reports, the authors found that butyrate directly inhibited the activity of nucleic HDAC in stem/progenitor cells and increased acetylation at both the histone H3K27 and H3K9 sites. This mechanism was further confirmed by using the prototypical pan-HDAC inhibitor trichostatin A, which also suppressed epithelial proliferation in zebrafish. Detailed mechanistic analysis using genome-wide chromatin-immunoprecipitation sequencing (Chip-seq) identifies that butyrate-induced suppression of stem/progenitor cell proliferation is mediated via Foxo3, a transcription factor that regulate cell-cycle genes. The reversal of butyrate effect upon pharmacological inhibition and genetic ablation of Foxo3 in colonic stem/progenitor cells further established Foxo3 as a master regulator of butyrate-mediated effects on cell proliferation.
Intriguingly, the anti-proliferative effect of butyrate was observed by Kaiko et al. to be dose-dependent, which is reversible at 1 mM but become pro-apoptotic at 3–10 mM. This lends further support to the emerging hypothesis that perhaps the doses of exposure and the colonic microenvironment (e.g., cell-type, crypt physiology) may underlie the discrepant anti- or pro-carcinogenesis and inflammatory effects of butyrate [as reviewed in (39)]. Indeed, the level of butyrate is much lower in the colonic crypts (50–800 µM) when compared to its levels in the lumen of proximal, medial and distal segments of mouse colons which are 3.5, 0.8 and 0.5 mM, respectively (40). The decreasing butyrate gradient from the top to the base of the crypt that is established, in part, by butyrate-metabolizing colonocytes can be perceived as a mechanism to reduce the degree of butyrate exposure to levels that can be tolerated by the stem cells. It would be interesting to measure the level of butyrate at the base of the crypt, and examine whether even the reduced butyrate level therein is still able to suppress the stem/progenitor cell proliferation.
From a mechanistic standpoint, it is reasonable to posit that the influence of butyrate on gut health may be primarily mediated through inhibiting HDAC activity. This notion is exemplified in a study by Alenghat et al. (41) which demonstrate that intestinal-specific deletion of epigenome-modifying enzyme histone HDAC-3 in mice resulted in compromised gut barrier function, reduced intestinal expression of antimicrobial defense genes, and eventually increased susceptibility to intestinal damage and inflammation. In a similar line, one could argue that persistent chronic exposure of butyrate in the gut may blunt HDAC activity in the colonocytes that might potentially increase the risk to develop IBD.
Despite all that, it seems rather unlikely that the ‘butyrate paradox’ would be resolved sooner, though we can be rest-assured that future studies that evaluate the possible factors (i.e., doses, gut physiology, microbiota dysbiosis) that dictate butyrate beneficial vs. detrimental effects could provide further insights into this area of research. Future studies should also explore whether or not long-term butyrate exposure reduces HDAC activity in the intestinal epithelial cells.
Reassessing the pre- and pro-biotics: to use or not to use
Manipulation of the human colonic microbiota through diet to improve healthy gut function and prevent systemic disease has been a long-term goal, and indeed, it has been shown that dietary complex carbohydrate intake has a major impact on the composition of the gut microbiota and its metabolic output. The use of pre- and pro-biotics as nutritional supplements are becoming increasingly popular with in the United States and European countries due to heightened awareness about the association between gut health and individual well-being. Researchers and nutritional supplement industries are enthusiastic to develop SCFA-rich prebiotics and probiotics that can produce more SCFA in the gut. Nowadays, several probiotic formulations are commercially available; particularly those favor SCFA production e.g., Bifidobacterium, in the gut (42). However, whether they meet their goals in the gut remains elusive. Use of probiotics in the patients on antibiotics, which substantially reduced gut microbial community, provide a steady relief however, its effect on healthy subjects require an exhaustive evaluation.
Perhaps the proverbial “one man’s meat is another man’s poison” may also apply to dietary soluble fiber, SCFAs and butyrate. There have been increasing reports cautioning that even beneficial probiotics in healthy hosts could become invading pathogens in a subset of individuals with intestinal inflammation (43). For instance, the use of probiotic formulation containing Bifidobacterium and Lactobacillus strains have been associated with a higher mortality rate in a patient cohort with predicted severe acute pancreatitis. Furthermore, the widely-accepted probiotic Lactobacillus spp., just like any other opportunistic bacteria, could become a pathogen and cause infections in immune-compromised patients (44) and also those with intestinal disorders (44,45). Given the very limited number of clinical trial data available on the health effects of probiotics during disease state [i.e., IBD, gut dysbiosis, small intestinal bacterial overgrowth (SIBO)] therefore, it is important to determine the long-term health effects of pre- and pro-biotics before prescribing them.
Future perspectives
This study suggests that the positive effect of butyrate on healthy colonocytes appeared, in part, due to its compulsion to act as metabolic barrier. Plethora of studies from both human and animal studies have suggested that administration of butyrate promotes healthy colon; yet this may not necessarily be due to the beneficial health effect of butyrate but instead may be the consequence of the body’s response to the presence of microbial metabolites. Many of the effects of gut microbiota on host health have been accompanied by gut microbial products (e.g., lipopolysaccharide, flagellin) and microbiota-derived metabolites such as SCFAs, branched chain fatty acids (BCFAs), lactate, ethanol, succinate, and α-keto acids, as well as sulfur compounds, which further play a role in regulating intestinal and extra-intestinal health. Future research should extensively explore the impact-specifically upon long-term exposure- of these metabolites on mammalian physiology to actualize the “bugs to drugs” concept.
Acknowledgments
Funding: M Vijay-Kumar is supported by grant from the National Institutes of Health R01 (DK097865). V Singh is supported by CCFA’s Research Fellowship Award. BS Yeoh is supported by NIH T32 (T32AI074551).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Fengbo Tan (Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaiko GE, Ryu SH, Koues OI, et al. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016;165:1708-20. [Crossref] [PubMed]
- Cummings JH, Pomare EW, Branch WJ, et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987;28:1221-7. [Crossref] [PubMed]
- den Besten G, van Eunen K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 2013;54:2325-40. [Crossref] [PubMed]
- Ng AN, de Jong-Curtain TA, Mawdsley DJ, et al. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol 2005;286:114-35. [Crossref] [PubMed]
- Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 2011;13:517-26. [Crossref] [PubMed]
- Willing BP, Dicksved J, Halfvarson J, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 2010;139:1844-1854.e1. [Crossref] [PubMed]
- Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis 2009;15:1183-9. [Crossref] [PubMed]
- Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014;63:1275-83. [Crossref] [PubMed]
- Kang S, Denman SE, Morrison M, et al. Dysbiosis of fecal microbiota in Crohn's disease patients as revealed by a custom phylogenetic microarray. Inflamm Bowel Dis 2010;16:2034-42. [Crossref] [PubMed]
- Zhang Q, Wu Y, Wang J, et al. Accelerated dysbiosis of gut microbiota during aggravation of DSS-induced colitis by a butyrate-producing bacterium. Sci Rep 2016;6:27572. [Crossref] [PubMed]
- Kuo SM, Chan WC, Hu Z. Wild-type and IL10-null mice have differential colonic epithelial gene expression responses to dietary supplementation with synbiotic Bifidobacterium animalis subspecies lactis and inulin. J Nutr 2014;144:245-51. [Crossref] [PubMed]
- Vijay-Kumar M, Sanders CJ, Taylor RT, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest 2007;117:3909-21. [PubMed]
- Singh V, Yeoh BS, Carvalho F, et al. Proneness of TLR5 deficient mice to develop colitis is microbiota dependent. Gut Microbes 2015;6:279-83. [Crossref] [PubMed]
- Singh V, Yeoh BS, Chassaing B, et al. Microbiota-inducible Innate Immune, Siderophore Binding Protein Lipocalin 2 is Critical for Intestinal Homeostasis. Cell Mol Gastroenterol Hepatol 2016;2:482-498.e6. [Crossref] [PubMed]
- Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap Adv Gastroenterol 2012;5:261-8. [Crossref] [PubMed]
- Gearry RB, Irving PM, Barrett JS, et al. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J Crohns Colitis 2009;3:8-14. [Crossref] [PubMed]
- Riggs MG, Whittaker RG, Neumann JR, et al. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature 1977;268:462-4. [Crossref] [PubMed]
- Candido EP, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell 1978;14:105-13. [Crossref] [PubMed]
- Gibson PR, Rosella O, Wilson AJ, et al. Colonic epithelial cell activation and the paradoxical effects of butyrate. Carcinogenesis 1999;20:539-44. [Crossref] [PubMed]
- Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014;40:128-39. [Crossref] [PubMed]
- Chang PV, Hao L, Offermanns S, et al. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 2014;111:2247-52. [Crossref] [PubMed]
- Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013;504:451-5. [Crossref] [PubMed]
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504:446-50. [Crossref] [PubMed]
- Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569-73. [Crossref] [PubMed]
- Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 2014;5:3611. [PubMed]
- den Besten G, Bleeker A, Gerding A, et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015;64:2398-408. [Crossref] [PubMed]
- Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 2013;4:1829. [Crossref] [PubMed]
- Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009;58:1509-17. [Crossref] [PubMed]
- Pajari AM, Rajakangas J, Päivärinta E, et al. Promotion of intestinal tumor formation by inulin is associated with an accumulation of cytosolic beta-catenin in Min mice. Int J Cancer 2003;106:653-60. [Crossref] [PubMed]
- Belcheva A, Irrazabal T, Robertson SJ, et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 2014;158:288-99. [Crossref] [PubMed]
- Kim MH, Kang SG, Park JH, et al. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013;145:396-406.e1-10.
- Park J, Goergen CJ. Chronically Elevated Levels of Short-Chain Fatty Acids Induce T Cell-Mediated Ureteritis and Hydronephrosis. J Immunol 2016;196:2388-400. [Crossref] [PubMed]
- Singh V, Chassaing B, Zhang L, et al. Microbiota-Dependent Hepatic Lipogenesis Mediated by Stearoyl CoA Desaturase 1 (SCD1) Promotes Metabolic Syndrome in TLR5-Deficient Mice. Cell Metab 2015;22:983-96. [Crossref] [PubMed]
- Perry RJ, Peng L, Barry NA, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016;534:213-7. [Crossref] [PubMed]
- Madesh M, Benard O, Balasubramanian KA. Increased phospholipase D activity in butyrate-induced differentiation of HT-29 cells. Cancer Lett 1998;132:141-6. [Crossref] [PubMed]
- Frohman MA. The phospholipase D superfamily as therapeutic targets. Trends Pharmacol Sci 2015;36:137-44. [Crossref] [PubMed]
- Jenkins GM, Frohman MA. Phospholipase D: a lipid centric review. Cell Mol Life Sci 2005;62:2305-16. [Crossref] [PubMed]
- Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr 2003;133:2485S-2493S. [PubMed]
- Sengupta S, Muir JG, Gibson PR. Does butyrate protect from colorectal cancer? J Gastroenterol Hepatol 2006;21:209-18. [Crossref] [PubMed]
- Donohoe DR, Collins LB, Wali A, et al. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell 2012;48:612-26. [Crossref] [PubMed]
- Alenghat T, Osborne LC, Saenz SA, et al. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature 2013;504:153-7. [Crossref] [PubMed]
- Sugahara H, Odamaki T, Fukuda S, et al. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci Rep 2015;5:13548. [Crossref] [PubMed]
- Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr 2006;83:1256-64; quiz 1446-7. [PubMed]
- Cannon JP, Lee TA, Bolanos JT, et al. Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur J Clin Microbiol Infect Dis 2005;24:31-40. [Crossref] [PubMed]
- Young RJ, Vanderhoof JA. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J Pediatr Gastroenterol Nutr 2004;39:436-7; author reply 437. [Crossref] [PubMed]


