
Activation of calcineurin in cancer: many paths, one hub
Introduction
Colorectal cancer is the third most diagnosed cancer and the fourth leading cause of cancer-related death worldwide, and the second leading cause of cancer-related deaths in developed countries (1). The worldwide incidence of colon cancer is predicted to rise by 60% within 15 years, in line with observations linking increased colon cancer incidence with increased industrial development (2). Intestinal inflammation is a risk factor for colorectal cancer: with inflammatory bowel disease significantly increasing the risk of colitis-associated colorectal cancer (3). Inflammatory signaling through STAT3 and NF-κB is also activated in colorectal tumors and cell lines (4,5). In their recent publication, Peuker et al. (6) identify calcineurin as a promoter of colorectal cancer growth through maintenance and proliferation of cancer stem cells. Calcineurin is a calcium dependent serine/threonine phosphatase that plays a central role in immunity, as demonstrated by the use of calcineurin inhibitors cyclosporine A and tacrolimus (FK506) as immunosuppressants (7).
Calcineurin is composed of two subunits, a catalytic subunit called calcineurin A (CNA) encoded by three separate genes (PPP3CA, PPP3CB and PPP3CC), and a regulatory subunit, calcineurin B (CNB; Cnb) encoded by two genes, PPP3R1 and PPP3R2, with the latter restricted to testis and brain. In the presence of elevated calcium, calmodulin binds to calcineurin, displacing the autoinhibitory domain from the active site, leading to activation of calcineurin and subsequent dephosphorylation of target proteins. Calcineurin substrates include transcription factors, proteins involved in cell cycle and apoptosis, cytoskeletal proteins, scaffold proteins, membrane channels and receptors (Table 1) (10).
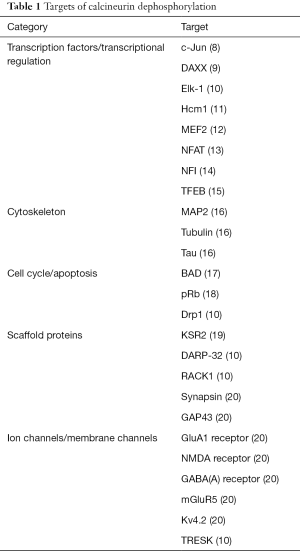
Full table
The best characterized calcineurin substrates are the nuclear factor of activated T cells (NFAT) transcription factors. Four of the five members of the NFAT protein family are regulated by calcium signaling: NFATc1 (NFAT2), NFATc2 (NFAT1), NFATc3 (NFAT4), and NFATc4 (NFAT3). In resting cells, NFAT is highly phosphorylated which precludes exposure of a nuclear localization sequence (21). Following dephosphorylation by calcineurin, NFAT is translocated to the nucleus where it regulates gene expression, including genes encoding cytokines in immune cells.
Peuker et al. (6) examine the contribution of calcineurin and its downstream target NFAT to intestinal tumor growth in both a genetic model and a colitis-associated model of colorectal cancer. By specifically deleting the regulatory B1 subunit of calcineurin (Cnb1, encoded by Ppp3r1) in the intestinal epithelial cells of the ApcMin/+ mouse model of colon cancer, they observe both fewer and smaller intestinal tumors. Similar results were obtained when mice carrying intestinal epithelial cell-specific deletion of Ppp3r1 were treated with colitis-inducing dextran sulfate along with the carcinogen azoxymethane. In particular, the authors noted that early stage lesions were reduced in their colorectal cancer models, and that the early lesions showed reduced epithelial proliferation and increased apoptosis. Peuker et al. (6) further showed that NFATc3 is the main NFAT expressed in normal intestinal epithelial cells, and that tumor formation is accompanied by calcineurin-dependent cytoplasmic to nuclear translocation of NFATc3. Intestinal epithelial cell-specific deletion of NFATc3 resulted in a phenotype similar to that of Ppp3r1−/− mice, albeit attenuated. The full phenotype was restored upon treatment of ApcMin/+ mice with a peptide that interferes with calcineurin-dependent activation of all NFATs.
Calcineurin in stem cell proliferation and differentiation
The intestinal epithelium is maintained by continuous renewal through a tightly regulated balance of intestinal stem cell proliferation and differentiation. In Drosophila, high cytoplasmic calcium concentration results in activation of calcineurin and downstream targets, triggering proliferation of intestinal stem cells (22). Silencing of either the regulatory subunit of calcineurin CanB2 or the catalytic subunit of calcineurin CanA1 in Drosophila significantly reduces stem cell proliferation, highlighting a crucial role for calcineurin in translating changes in calcium signaling to proliferation of intestinal stem cells.
Paradoxically, in addition to a role in cell proliferation, calcineurin also promotes differentiation. Calcineurin-NFAT signaling is necessary for lineage specification in embryonic stem cells, triggering the transition of these stem cells from self-renewal to differentiation (23). Calcineurin-NFAT signaling also initiates skeletal muscle differentiation (24), alveolar specification of adult lung stem cells (25), terminal differentiation of osteoclasts (26), and stem cell quiescence in keratinocytes (27). In the cardiovascular system, calcineurin is important for cardiomyocyte maturation, valve formation, and vascular development. Loss of calcineurin results in heart defects and reduced proliferation, whereas calcineurin is necessary for hypertrophic response in adult cardiomyocytes (28,29). In the brain, calcineurin is highly expressed in neurons, and plays an important role in the modulation of synaptic transmission (20). Calcineurin activity is also required for neural induction in the developing embryo through dephosphorylation and inactivation of Smad1/5 to antagonize signaling through the bone morphogenetic protein (BMP) (30).
The immunosuppressive activity of the calcineurin inhibitor cyclosporine A was first described in 1976, and calcineurin inhibitors have been widely used as immunosuppressants since the mid-80’s (31). The study of calcineurin inhibitors revealed a vital role for calcineurin in regulating T cell development and activation through the NFAT family (32-34). In T cells, binding of the T-cell receptor results in release of calcium from intracellular stores, which activates sustained Ca2+ entry through Ca2+ release-activated Ca2+ (CRAC) channels (35). As a result, calcineurin is activated and NFAT is dephosphorylated and shuttles to the nucleus where it binds to the promoters of T cell-activated proteins including interleukin 2 (IL-2) and interleukin 3 (IL-3) to induce transcription (36,37). The immunosuppressant action of cyclosporine A and tacrolimus are mediated by inhibiting dephosphorylation of NFAT by calcineurin in immune cells, especially T cells (7,34,37,38).
Calcineurin in cancer
Chronic use of immunosuppressants such as the calcineurin inhibitors cyclosporine A and tacrolimus increases cancer incidence (39). Overall, the transplant population has a two-fold increased risk of cancer, with much higher increases for specific cancers, including nonmelanoma skin cancer and virally linked cancers (40). This increased risk of cancer is linked to three primary mechanisms: (I) increased risk of virally driven malignancy due to immunosuppression; (II) impaired immunosurveillance of transformed cells; and (III) specific effects of drugs used for immunosuppression (39). The first two are directly related to disruption of the immune system. Thus, calcineurin inhibitors can directly promote tumorigenesis in an autonomous manner through modulation of the immune system (41-43).
In apparent contradiction, activation of calcineurin and its downstream targets also increases tumorigenic potential. As observed by Peuker et al. and others, calcineurin and downstream signalling pathways are activated in colorectal cancer tumors and cell lines, and inhibition of calcineurin decreases cancer stem cell survival and proliferation (6,44). Similarly, calcineurin is activated in breast cancer, specifically in triple negative breast cancer, and promotes migration and invasion in vitro and growth and metastasis in vivo (45,46). Analogous findings by others support a pro-tumorigenic role for calcineurin signaling in lung, prostate, bladder, ovarian, pancreatic, and liver cancer, as well as glioblastoma, melanoma and leukemia (14,47-55).
Studies addressing calcineurin activation have focused principally on dephosphorylation and activation of NFAT and NFAT transcriptional targets. NFAT in and of itself is constitutively activated or overexpressed in numerous cancers and can contribute to cancer development and progression (56). Additional calcineurin substrates including myocyte enhancer factor 2 (MEF2), kinase suppressor of ras 2 (KSR2), DAXX, c-Jun and Nuclear Factor I (NFI) have all been shown to have pro-tumorigenic roles (8,9,14,57-60). For example, c-Jun is stabilized by calcineurin dephosphorylation in cervical cancers compared to normal tissue, thereby increasing c-Jun dependent gene expression (8). Calcineurin has a similar effect on the transcription factor NFI, with dephosphorylation by calcineurin increasing its transcription regulatory activity (14).
Activation of calcineurin
Calcineurin can be activated by a variety of mechanisms (Figure 1). The newly discovered mechanism reported by Peuker et al. (6) appears to be specific to intestinal cancers, and is driven by changes in gut microbiota stratification and composition. This change in microbiota activates toll-like receptor (TLR) signaling which in turn induces calcium entry and calcineurin activation. Although the exact mechanism of action of the gut microbiota was not addressed in their paper, Peuker and colleagues did observe differences in microbial community structure between intestinal tumors and normal intestinal mucosa. The discovery that calcineurin can be activated by alterations in bacterial communities is exciting and a major step forward in our understanding of calcineurin’s importance in tumor formation.
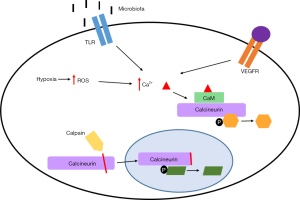
The mechanisms driving calcineurin activation in other cancers is less clear. It is a well-known fact that the inflammatory response is activated in many cancers, and that chronic inflammation is a risk factor for tumor development (3). There is also ample evidence showing that inflammation activates calcineurin activity (61-63). Thus, chronic inflammation triggered by environmental factors such as chronic infection, inhaled pollutants, and dietary factors may activate calcineurin activity, thereby contributing to tumor initiation and growth. Necrosis resulting from tumor growth would further contribute to the inflammatory response (64). A well-characterized receptor implicated in calcineurin activation is the vascular endothelial growth factor receptor (VEGFR) whose activation increases cytosolic calcium in endothelial cells to regulate tumor angiogenesis and metastasis (49,65). Hypoxia can also activate calcineurin through increased intracellular calcium by activation of CRAC channels (66), and calcium signaling through CRAC channels promotes proliferation, migration and metastasis in a number of tumors (67,68).
Calcineurin activity is modulated by a number of factors in addition to intracellular calcium including interaction with regulatory factors, subcellular localization, and intramolecular cleavage. Regulators of calcineurin (RCAN1-4) can bind to and inhibit calcineurin activity (69-71). RCANs bind calcineurin at the same site as NFAT and other substrates, and one mechanism of action is via competition for binding (72). Increased expression of RCAN1 (also known as Down’s syndrome candidate region-1, DSCR1) inhibits tumor growth in mice, and increased copies of the DSCR1 gene contributes to decreased cancer rates in Down’s syndrome (73)
Calcineurin is predominantly cytosolic in unstimulated cells (74,75). However, in response to calcium signaling, calcineurin can translocate to the nucleus to interact with target substrates (76). A nuclear localization signal (NLS) in the catalytic domain of CNA is necessary for nuclear import of activated calcineurin via importin β1, with a nuclear export signal (NES) located in the C-terminus of CNA (Figure 2A). The autoinhibitory domain of CNA regulates nuclear import and export by masking the NLS in inactive calcineurin in addition to blocking the catalytic site (10,75). Subcellular localization is also mediated by interaction with targeting proteins, with the AKAP 79/150 scaffold shown to anchor calcineurin at distinct subcellular locations (77).
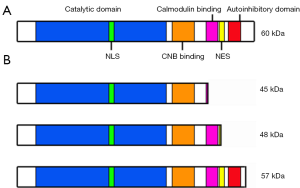
The autoinhibitory domain of CNA is located in the C-terminal region (75). In response to stress, calcineurin can be cleaved by the Ca2+-dependent cysteine-protease calpain, resulting in three main products of 45 kDa, 48 kDa and 57 kDa (Figure 2B). The autoinhibitory domain is missing from the 45 kDa and 48 kDa isoforms, resulting in constitutively active products that localize to the nucleus (75,78). Cleaved forms of CNA have been observed in cardiac hypertrophy, in the brains of Alzheimer’s disease patients, in response to neurotoxicity, and in a glaucoma model (75,79-81). In Alzheimer’s disease brains, the 57 kDa cleaved form of CNA which retains the autoinhibitory domain, still requires Ca2+ and calmodulin for its activation, but shows increased activity compared to the 60 kDa uncleaved form of CNA (80). The 57 kDa cleaved form of CNA has also been detected in malignant glioma cells, where it localizes to the nucleus, and regulates dephosphorylation and activation of NFI (14). NFI activates expression of the pro-migratory gene fatty acid-binding protein 7 (FABP7) and represses transcription of the tumor suppressors p21 and p53 in malignant glioma (82-85). NFAT is also expressed and localizes to the nucleus in these tumors (50).
Expression, activation, and cleavage of calcineurin are not commonly studied in cancer. Direct measurement of calcineurin activation is difficult: in vitro assays of calcineurin activity do not clearly correlate with endogenous activity, and antibodies to calcineurin may not detect cleaved forms. Thus, calcineurin activity is commonly measured by phosphorylation, nuclear localization, or transcriptional activity of NFAT. Peuker et al. (6) do not directly examine calcineurin expression, activity, and subcellular localization in their study, but use NFAT nuclear localization in tissue samples as a readout of calcineurin activity. Consequently, the localization and forms of calcineurin expressed in their intestinal tumor models remain unclear. To date, cleavage of CNA in cancer has only been clearly demonstrated in malignant glioma (14). However, immunoblotting of cervical tissue lysates with anti-CNA antibody reveals differences in migration of CNA between normal and tumor tissues in a subset of cases (8). Banding patterns consistent with CNA cleavage are also observed in T-ALL cells (86). Furthermore, CNA has been shown to localize to the nucleus in small cell lung cancer, although immunoblotting was not carried out to examine CNA banding patterns (87). These combined data suggest that calcineurin can be activated by CNA cleavage under different pathological conditions, including cancer. It will therefore be important to examine the subcellular localization of calcineurin as well as detect the forms of CNA present in the intestinal cancers studied by Peuker and colleagues.
Cleavage of CNA and nuclear localization of calcineurin in response to pathogenic stimuli also modulate substrate dephosphorylation. Nuclear localization of calcineurin alters substrate accessibility, resulting in decreased dephosphorylation of cytoskeletal and membrane-associated substrates, thereby modulating downstream signaling pathways. Subcellular localization of calcineurin has also been shown to regulate dephosphorylation of the pro-apoptotic Bcl-2 family member BAD (17) and NFAT (88).
Targeting calcineurin activation for the treatment of cancer
Numerous kinases and their phosphorylated substrates play critical roles in cancer formation and progression. Convergence of varied inputs resulting in calcineurin activation in different types of cancers implies a central role for the calcineurin phosphatase and its dephosphorylated substrates in malignancy. Activation of calcineurin by altered microbiota stratification in colorectal cancer builds on previous observations of calcineurin activation in cancer. Activation through a variety of mechanisms in both solid tumors and lymphoid malignancies further underlines the importance of this pathway in cancer, and suggests that dephosphorylation of calcineurin substrates transcends tissue-specific factors associated with tumor progression.
Preliminary studies have examined the use of calcineurin inhibitors as potential anti-cancer agents. Cyclosporine A treatment induced apoptosis in lymphoma and leukemia cell lines, and treatment of mouse models of T-cell acute lymphoblastic leukemia with cyclosporine A or tacrolimus increased mouse survival and resulted in rapid tumor clearance (54). Similarly, cyclosporine A and tacrolimus decreased proliferation and migration in both bladder and prostate tumor cells in vitro, and bladder and prostate xenografts in vivo (89,90). Tacrolimus treatment in a mouse model of breast cancer reduced tumor growth, and angiogenesis within these tumors in vivo, and decreased migration of both breast cancer and endothelial cells in vitro (91). In patients with acute myeloid leukemia who have an internal tandem duplication of Fms-related tyrosine kinase receptor 3 (FLT3), a retrospective analysis shows a promising correlation between inclusion of cyclosporine A in salvage therapy and increased survival (92).
As alluded to earlier, there are caveats to targeting calcineurin for cancer treatment. For example, in breast cancer, calcineurin can interact with the plasma membrane calcium ATPase 2 (PMCA2), which can sequester calcineurin to the membrane, reducing activation of the calcineurin-NFAT pathway. However, specific disruption of the PMCA2-calcineurin interaction with a small peptide increases NFAT activity, and reduces cell viability in breast cancer cell lines, resulting in increased apoptosis and sensitivity to paclitaxel (93). Treatment with calcineurin inhibitors can also increase the risk of cancer, as observed in transplant patients. Furthermore, cyclosporine A itself is also directly implicated in tumor growth as it can increase TGFβ production (41), activate Ras (43), and suppress PTEN expression, increasing activation of AKT (42).
More specific targeting of specific components of calcineurin activation may alleviate issues associated with long term treatment with cyclosporine A and tacrolimus. For example, inhibitors targeting interaction with specific substrates, or specific sets of substrates may help to focus growth inhibitory effects on cancer cells. If additional work in the field demonstrates that altered cleavage and/or nuclear localization of calcineurin is a frequent event in cancer, altering cleavage or nuclear localization would present a tumor-specific way to target activated calcineurin in cancer cells, while preserving normal immune function.
In conclusion, phosphorylation represents one of the most, if not the most, well-studied and widespread mechanism regulating protein function in both normal and cancer cells. There is emerging evidence suggesting that dephosphorylation of proteins by the phosphatase calcineurin may also play a critical role in tumor formation and progression. Calcineurin’s activity is regulated by surprisingly diverse mechanisms, ranging from the gut microbiota and inflammation, to intramolecular cleavage and subcellular localization. Further work on the role of calcineurin in cancer may reveal a fundamental pathway that is commonly altered to promote cancer formation.
Acknowledgments
Funding: This work was supported by a grant from the Canadian Institutes of Health Research (grant number 130314).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Fengbo Tan (Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.09.30). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Terzić J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology 2010;138:2101-2114.e5. [Crossref] [PubMed]
- De Simone V, Franze E, Ronchetti G, et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015;34:3493-503. [Crossref] [PubMed]
- Peuker K, Muff S, Wang J, et al. Epithelial calcineurin controls microbiota-dependent intestinal tumor development. Nat Med 2016;22:506-15. [Crossref] [PubMed]
- Liu J, Farmer JD Jr, Lane WS, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991;66:807-15. [Crossref] [PubMed]
- Huang CC, Wang JM, Kikkawa U, et al. Calcineurin-mediated dephosphorylation of c-Jun Ser-243 is required for c-Jun protein stability and cell transformation. Oncogene 2008;27:2422-9. [Crossref] [PubMed]
- Michod D, Bartesaghi S, Khelifi A, et al. Calcium-dependent dephosphorylation of the histone chaperone DAXX regulates H3.3 loading and transcription upon neuronal activation. Neuron 2012;74:122-35. [Crossref] [PubMed]
- Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol 2011;21:91-103. [Crossref] [PubMed]
- Arsenault HE, Roy J, Mapa CE, et al. Hcm1 integrates signals from Cdk1 and calcineurin to control cell proliferation. Mol Biol Cell 2015;26:3570-7. [Crossref] [PubMed]
- Shalizi A, Gaudilliere B, Yuan Z, et al. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science 2006;311:1012-7. [Crossref] [PubMed]
- Jain J, McCaffrey PG, Miner Z, et al. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature 1993;365:352-5. [Crossref] [PubMed]
- Brun M, Glubrecht DD, Baksh S, et al. Calcineurin regulates nuclear factor I dephosphorylation and activity in malignant glioma cell lines. J Biol Chem 2013;288:24104-15. [Crossref] [PubMed]
- Medina DL, Di Paola S, Peluso I, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 2015;17:288-99. [Crossref] [PubMed]
- Goto S, Yamamoto H, Fukunaga K, et al. Dephosphorylation of microtubule-associated protein 2, tau factor, and tubulin by calcineurin. J Neurochem 1985;45:276-83. [Crossref] [PubMed]
- Wang HG, Pathan N, Ethell IM, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 1999;284:339-43. [Crossref] [PubMed]
- Qiu Z, Ghosh A. A calcium-dependent switch in a CREST-BRG1 complex regulates activity-dependent gene expression. Neuron 2008;60:775-87. [Crossref] [PubMed]
- Dougherty MK, Ritt DA, Zhou M, et al. KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals. Mol Cell 2009;34:652-62. [Crossref] [PubMed]
- Baumgärtel K, Mansuy IM. Neural functions of calcineurin in synaptic plasticity and memory. Learn Mem 2012;19:375-84. [Crossref] [PubMed]
- Mancini M, Toker A. NFAT proteins: emerging roles in cancer progression. Nat Rev Cancer 2009;9:810-20. [Crossref] [PubMed]
- Deng H, Gerencser AA, Jasper H. Signal integration by Ca(2+) regulates intestinal stem-cell activity. Nature 2015;528:212-7. [Crossref] [PubMed]
- Li X, Zhu L, Yang A, et al. Calcineurin-NFAT signaling critically regulates early lineage specification in mouse embryonic stem cells and embryos. Cell Stem Cell 2011;8:46-58. [Crossref] [PubMed]
- Tu MK, Levin JB, Hamilton AM, et al. Calcium signaling in skeletal muscle development, maintenance and regeneration. Cell Calcium 2016;59:91-7. [Crossref] [PubMed]
- Lee JH, Bhang DH, Beede A, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 2014;156:440-55. [Crossref] [PubMed]
- Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 2002;3:889-901. [Crossref] [PubMed]
- Horsley V, Aliprantis AO, Polak L, et al. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 2008;132:299-310. [Crossref] [PubMed]
- Schulz RA, Yutzey KE. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol 2004;266:1-16. [Crossref] [PubMed]
- Maillet M, Davis J, Auger-Messier M, et al. Heart-specific deletion of CnB1 reveals multiple mechanisms whereby calcineurin regulates cardiac growth and function. J Biol Chem 2010;285:6716-24. [Crossref] [PubMed]
- Cho A, Tang Y, Davila J, et al. Calcineurin signaling regulates neural induction through antagonizing the BMP pathway. Neuron 2014;82:109-24. [Crossref] [PubMed]
- Karam S, Wali RK. Current State of Immunosuppression: Past, Present, and Future. Crit Rev Eukaryot Gene Expr 2015;25:113-34. [Crossref] [PubMed]
- Liu J, Albers MW, Wandless TJ, et al. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry 1992;31:3896-901. [Crossref] [PubMed]
- Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 1992;357:695-7. [Crossref] [PubMed]
- Shaw KT, Ho AM, Raghavan A, et al. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT1 in stimulated immune cells. Proc Natl Acad Sci U S A 1995;92:11205-9. [Crossref] [PubMed]
- Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol 2005;5:472-84. [Crossref] [PubMed]
- Serfling E, Berberich-Siebelt F, Chuvpilo S, et al. The role of NF-AT transcription factors in T cell activation and differentiation. Biochim Biophys Acta 2000;1498:1-18. [Crossref] [PubMed]
- Hogan PG, Chen L, Nardone J, et al. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 2003;17:2205-32. [Crossref] [PubMed]
- Matsuda S, Shibasaki F, Takehana K, et al. Two distinct action mechanisms of immunophilin-ligand complexes for the blockade of T-cell activation. EMBO Rep 2000;1:428-34. [Crossref] [PubMed]
- Sherston SN, Carroll RP, Harden PN, et al. Predictors of cancer risk in the long-term solid-organ transplant recipient. Transplantation 2014;97:605-11. [Crossref] [PubMed]
- Engels EA, Pfeiffer RM, Fraumeni JF Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306:1891-901. [Crossref] [PubMed]
- Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature 1999;397:530-4. [Crossref] [PubMed]
- Han W, Ming M, He TC, et al. Immunosuppressive cyclosporin A activates AKT in keratinocytes through PTEN suppression: implications in skin carcinogenesis. J Biol Chem 2010;285:11369-77. [Crossref] [PubMed]
- Datta D, Contreras AG, Basu A, et al. Calcineurin inhibitors activate the proto-oncogene Ras and promote protumorigenic signals in renal cancer cells. Cancer Res 2009;69:8902-9. [Crossref] [PubMed]
- Masuo T, Okamura S, Zhang Y, et al. Cyclosporine A inhibits colorectal cancer proliferation probably by regulating expression levels of c-Myc, p21(WAF1/CIP1) and proliferating cell nuclear antigen. Cancer Lett 2009;285:66-72. [Crossref] [PubMed]
- Jauliac S, Lopez-Rodriguez C, Shaw LM, et al. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol 2002;4:540-4. [Crossref] [PubMed]
- Quang CT, Leboucher S, Passaro D, et al. The calcineurin/NFAT pathway is activated in diagnostic breast cancer cases and is essential to survival and metastasis of mammary cancer cells. Cell Death Dis 2015;6:e1658 [Crossref] [PubMed]
- Gachet S, Genesca E, Passaro D, et al. Leukemia-initiating cell activity requires calcineurin in T-cell acute lymphoblastic leukemia. Leukemia 2013;27:2289-300. [Crossref] [PubMed]
- Shoshan E, Braeuer RR, Kamiya T, et al. NFAT1 Directly Regulates IL8 and MMP3 to Promote Melanoma Tumor Growth and Metastasis. Cancer Res 2016;76:3145-55. [Crossref] [PubMed]
- Minami T, Jiang S, Schadler K, et al. The calcineurin-NFAT-angiopoietin-2 signaling axis in lung endothelium is critical for the establishment of lung metastases. Cell Rep 2013;4:709-23. [Crossref] [PubMed]
- Tie X, Han S, Meng L, et al. NFAT1 is highly expressed in, and regulates the invasion of, glioblastoma multiforme cells. PLoS One 2013;8:e66008 [Crossref] [PubMed]
- Xu W, Gu J, Ren Q, et al. NFATC1 promotes cell growth and tumorigenesis in ovarian cancer up-regulating c-Myc through ERK1/2/p38 MAPK signal pathway. Tumour Biol 2016;37:4493-500. [Crossref] [PubMed]
- Wang S, Kang X, Cao S, et al. Calcineurin/NFATc1 pathway contributes to cell proliferation in hepatocellular carcinoma. Dig Dis Sci 2012;57:3184-8. [Crossref] [PubMed]
- Manda KR, Tripathi P, Hsi AC, et al. NFATc1 promotes prostate tumorigenesis and overcomes PTEN loss-induced senescence. Oncogene 2016;35:3282-92. [Crossref] [PubMed]
- Medyouf H, Alcalde H, Berthier C, et al. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat Med 2007;13:736-41. [Crossref] [PubMed]
- Buchholz M, Schatz A, Wagner M, et al. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J 2006;25:3714-24. [Crossref] [PubMed]
- Qin JJ, Nag S, Wang W, et al. NFAT as cancer target: mission possible? Biochim Biophys Acta 2014;1846:297-311.
- Pon JR, Marra MA. MEF2 transcription factors: developmental regulators and emerging cancer genes. Oncotarget 2016;7:2297-312. [PubMed]
- Flavell SW, Cowan CW, Kim TK, et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 2006;311:1008-12. [Crossref] [PubMed]
- Puto LA, Brognard J, Hunter T. Transcriptional Repressor DAXX Promotes Prostate Cancer Tumorigenicity via Suppression of Autophagy. J Biol Chem 2015;290:15406-20. [Crossref] [PubMed]
- Fernandez MR, Henry MD, Lewis RE. Kinase suppressor of Ras 2 (KSR2) regulates tumor cell transformation via AMPK. Mol Cell Biol 2012;32:3718-31. [Crossref] [PubMed]
- Furman JL, Norris CM. Calcineurin and glial signaling: neuroinflammation and beyond. J Neuroinflammation 2014;11:158. [Crossref] [PubMed]
- Musson RE, Smit NP. Regulatory mechanisms of calcineurin phosphatase activity. Curr Med Chem 2011;18:301-15. [Crossref] [PubMed]
- Minami T. Calcineurin-NFAT activation and DSCR-1 auto-inhibitory loop: how is homoeostasis regulated? J Biochem 2014;155:217-26. [Crossref] [PubMed]
- Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol 2004;4:641-8. [Crossref] [PubMed]
- Suehiro J, Kanki Y, Makihara C, et al. Genome-wide approaches reveal functional vascular endothelial growth factor (VEGF)-inducible nuclear factor of activated T cells (NFAT) c1 binding to angiogenesis-related genes in the endothelium. J Biol Chem 2014;289:29044-59. [Crossref] [PubMed]
- Mungai PT, Waypa GB, Jairaman A, et al. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol 2011;31:3531-45. [Crossref] [PubMed]
- Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiol Rev 2015;95:1383-436. [Crossref] [PubMed]
- Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 2009;15:124-34. [Crossref] [PubMed]
- Rothermel B, Vega RB, Yang J, et al. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem 2000;275:8719-25. [Crossref] [PubMed]
- Fuentes JJ, Genesca L, Kingsbury TJ, et al. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet 2000;9:1681-90. [Crossref] [PubMed]
- Kingsbury TJ, Cunningham KW. A conserved family of calcineurin regulators. Genes Dev 2000;14:1595-604. [PubMed]
- Martínez-Martínez S, Genescà L, Rodríguez A, et al. The RCAN carboxyl end mediates calcineurin docking-dependent inhibition via a site that dictates binding to substrates and regulators. Proc Natl Acad Sci U S A 2009;106:6117-22. [Crossref] [PubMed]
- Baek KH, Zaslavsky A, Lynch RC, et al. Down's syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature 2009;459:1126-30. [Crossref] [PubMed]
- Shibasaki F, McKeon F. Calcineurin functions in Ca(2+)-activated cell death in mammalian cells. J Cell Biol 1995;131:735-43. [Crossref] [PubMed]
- Hallhuber M, Burkard N, Wu R, et al. Inhibition of nuclear import of calcineurin prevents myocardial hypertrophy. Circ Res 2006;99:626-35. [Crossref] [PubMed]
- Shibasaki F, Price ER, Milan D, et al. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature 1996;382:370-3. [Crossref] [PubMed]
- Coghlan VM, Perrino BA, Howard M, et al. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science 1995;267:108-11. [Crossref] [PubMed]
- Burkard N, Becher J, Heindl C, et al. Targeted proteolysis sustains calcineurin activation. Circulation 2005;111:1045-53. [Crossref] [PubMed]
- Wu HY, Tomizawa K, Oda Y, et al. Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem 2004;279:4929-40. [Crossref] [PubMed]
- Liu F, Grundke-Iqbal I, Iqbal K, et al. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J Biol Chem 2005;280:37755-62. [Crossref] [PubMed]
- Huang W, Fileta JB, Dobberfuhl A, et al. Calcineurin cleavage is triggered by elevated intraocular pressure, and calcineurin inhibition blocks retinal ganglion cell death in experimental glaucoma. Proc Natl Acad Sci U S A 2005;102:12242-7. [Crossref] [PubMed]
- Lee JS, Xiao J, Patel P, et al. A novel tumor-promoting role for nuclear factor IA in glioblastomas is mediated through negative regulation of p53, p21, and PAI1. Neuro Oncol 2014;16:191-203. [Crossref] [PubMed]
- Brun M, Coles JE, Monckton EA, et al. Nuclear factor I regulates brain fatty acid-binding protein and glial fibrillary acidic protein gene expression in malignant glioma cell lines. J Mol Biol 2009;391:282-300. [Crossref] [PubMed]
- Bisgrove DA, Monckton EA, Packer M, et al. Regulation of brain fatty acid-binding protein expression by differential phosphorylation of nuclear factor I in malignant glioma cell lines. J Biol Chem 2000;275:30668-76. [Crossref] [PubMed]
- Mita R, Coles JE, Glubrecht DD, et al. B-FABP-expressing radial glial cells: the malignant glioma cell of origin? Neoplasia 2007;9:734-44. [Crossref] [PubMed]
- Tosello V, Bordin F, Yu J, et al. Calcineurin and GSK-3 inhibition sensitizes T-cell acute lymphoblastic leukemia cells to apoptosis through X-linked inhibitor of apoptosis protein degradation. Leukemia 2016;30:812-22. [Crossref] [PubMed]
- Liu Y, Zhang Y, Min J, et al. Calcineurin promotes proliferation, migration, and invasion of small cell lung cancer. Tumour Biol 2010;31:199-207. [Crossref] [PubMed]
- Shibasaki F, Kondo E, Akagi T, et al. Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature 1997;386:728-31. [Crossref] [PubMed]
- Kawahara T, Kashiwagi E, Ide H, et al. Cyclosporine A and tacrolimus inhibit bladder cancer growth through down-regulation of NFATc1. Oncotarget 2015;6:1582-93. [Crossref] [PubMed]
- Kawahara T, Kashiwagi E, Ide H, et al. The role of NFATc1 in prostate cancer progression: cyclosporine A and tacrolimus inhibit cell proliferation, migration, and invasion. Prostate 2015;75:573-84. [Crossref] [PubMed]
- Siamakpour-Reihani S, Caster J, Bandhu Nepal D, et al. The role of calcineurin/NFAT in SFRP2 induced angiogenesis--a rationale for breast cancer treatment with the calcineurin inhibitor tacrolimus. PLoS One 2011;6:e20412 [Crossref] [PubMed]
- Metzelder SK, Michel C, von Bonin M, et al. NFATc1 as a therapeutic target in FLT3-ITD-positive AML. Leukemia 2015;29:1470-7. [Crossref] [PubMed]
- Baggott RR, Mohamed TM, Oceandy D, et al. Disruption of the interaction between PMCA2 and calcineurin triggers apoptosis and enhances paclitaxel-induced cytotoxicity in breast cancer cells. Carcinogenesis 2012;33:2362-8. [Crossref] [PubMed]


