
The MMP-7-181A/G gene polymorphism is a prognostic indicator in patients with gastric cancer
Introduction
Gastric cancer still ranks as one of the most prevalent malignancies worldwide with remarkably high fatality (1). And Cancer Statistics in China, 2015 indicated that an estimated 679,100 new gastric cancer cases and 498,000 gastric cancer deaths would occur in China in 2015 (2). The prognosis of gastric cancer is not reliably predicted using current clinicopathological indicators. Molecular markers to optimize the individualized therapy and predict the prognosis of this malignant disease are under extensive investigation. Genetic markers of RFLP (restriction fragment length polymorphism), microsatellite and SNP (single nucleotide polymorphism) comprise three molecular genetics tools for the study of disease. Present researches have showed genetic polymorphisms confer susceptibility both to several types of cancer and to malignant biological features (3,4). The role of these genetic polymorphisms in cancer susceptibility has been most extensively evaluated for isozymes of cytochrome P450 (CYP1A1, CYP2D6, and CYP2E1), N-acetyltransferase (NAT1 and NAT2), glutathione S-transferase (GSTM1, GSTT1, and GSTP1), microsomal epoxide hydrolase, and NAD(P)H:quinone oxidoreductase (5). And early monogenic association studies focusing on candidate genes with strong biological hypothesis have demonstrated an increased risk of cancers associated with polymorphisms in genes involved in cell cycle control, carcinogen metabolism, DNA (deoxyribonucleic acid) repair, apoptosis, inflammation and epigenetic regulation (5,6). Thus, they may have prognostic value.
MMPs regulate multiple biological processes: they can degrade extracellular matrix proteins, are implicated in connective tissue destruction and remodeling associated with cancer invasion and metastasis, contribute to cartilage destruction in arthritis and are involved in atherosclerotic plaque rupture (7-10). Genetic polymorphisms in the promoter of MMP genes have allele-specific effects on transcriptional activities, are associated with susceptibility to multiple cancers and confer aggressive or metastatic biological features. These polymorphisms may serve as markers to predict the susceptibility and prognosis of cancers and have potential to facilitate the diagnosis, therapeutic decisions and prognostication of cancer patients (11-30).
Recent studies found that the MMP-7 promoter region near -181 in the A/G polymorphism is functional and closely related to gastric cancer risk, which revealed that this gene polymorphism might serve as a susceptibility marker for gastric cancer. Among the multiple gene polymorphisms of MMPs and TIMPs, the MMP-7-181A/G polymorphism is one of the most differentially distributed between gastric cancer patients and healthy controls. Functional analysis in vitro has shown that nuclear proteins bind with higher affinity to the -181G allele than to the -181A allele and promoter activity variation of the -181G allele was about 2–3 times than that of the -181A allele, which may induce elevation of MMP-7 mRNA transcription and subsequently increase its protein levels, so individuals with GG genotype may have a higher risk of the gastric cancer than with GA genotype. Furthermore, the MMP-7-181A/G polymorphism is significantly correlated with helicobacter pylori (Hp) infection, which is a key aetiological factor in gastric cancer. The G allele may serve as a promoting factor for tumor growth and lymph node invasion. Thus, it may have prognostic value (31-34).
In this study, we detected the polymorphism of MMP-7-181A/G in lymph node tissue samples collected from 210 gastric carcinoma patients with no confirmed metastasis. Subsequently, we analyzed the association of the potential molecular marker with clinicopathological characteristics and prognosis of patients, intending to investigate the potential role of the MMP-7-181A/G polymorphism as a prognosticator.
Methods
Subjects
Gastric cancer patients (n=210) who underwent clinical surgery were recruited from the Beijing Cancer Hospital between January 1999 and December 2000. Patients were excluded if without adequate histological specimens or lacking clinical information. Lymph nodes tissue samples from the patients with pathological symptoms but no confirmed signs of metastasis were suitable for DNA extraction and subsequently collected. None of the enrolled patients received neo-adjuvant chemo-radiotherapy. The study was conducted under the assent of the ethics and research committee of Peking University School of Oncology. Tumor staging was according to the 2002 sixth edition of AJCC/UICC TNM staging criteria for gastric cancer. All patients were followed up at regular intervals of 6 months after surgery with a minimum of 5 years of follow-up. Tumor recurrence was clinically based on the reappearance of the tumor after curative surgery. The overall survival time was evaluated from the surgery date to the last visit or death.
Genotyping
Genomic DNA was extracted from paraffin-embedded tissue sections (three to five 10 µm sections) using a Genomic DNA Extraction Kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s protocol. The MMP-7-181A/G polymorphism was identified by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). The primers used to amplify MMP-7 were the following: forward primer TGG TAC CAT AAT GTC CTG AAT G and reverse primer TCG TTA TTG GCA GGA AGC ACA CAA TGAATT, as described by Jormsjö et al. (33). The reaction conditions used were an initial denaturation at 94 °C for 5 minutes, followed by 35 step cycles of denaturation at 94 °C for 30 seconds, annealing at 62 °C for 30 seconds, and extension at 72 °C for 30 seconds, followed by a terminal extension time of 5 minutes. PCR products were digested with the EcoRI restriction enzyme (New England Biolabs, Inc.) for 2 hours at 37 °C. The digestion products were then resolved on a 3% agarose gel containing ethidium bromide. The A/A variant was identified by a single band (150 bp), and the A/G variant displayed three bands (150, 120, and 30 bp; Figure 1).

Statistical analysis
All statistical analyses were performed using SPSS version 10.0 software (SPSS Inc., Chicago, IL, USA). Associations between the MMP-7-181 genotype and clinicopathologic characteristics were analyzed using Pearson’s χ2 test. Cumulative survival probability as a function of time was estimated using the Kaplan-Meier method, and the statistical significance of difference between survival curves was evaluated by the log-rank test. Differences with a two-sided P value of 0.05 or less were considered to be statistically significant. Cox’s proportional hazards modeling of the factors including the MMP-7-181A/G genotype and established clinicopathologic characteristics which may potentially related to survival was performed in order to identify which factors might have a significant influence on survival.
Results
Patient characteristics
Our study group consisted of 142 male and 68 female patients, the median age of the study population was 56 years (range, 25–78 years). There were 34 cases of patients were classified as stage I, 52 cases of stage II, 64 cases of stage III and 60 cases of stage IV. Among them 133 cases were poorly differentiated, 43 cases were moderately differentiated and 34 cases were well differentiated. According to the Lauren classification, 70 tumors were intestinal, 97 were diffuse and the remaining 43 were mixed. The follow-up period for survivors ranged from 2 to 120 months (median: 34 months). The 5-year overall survival rate for the entire study population was 43.7% with 92.7% in stage I, 75.2% in stage II, 34.3% in stage III and 13.5% in stage IV patients.
The association between MMP-7-181 polymorphic variants and clinicopathologic characteristics
Of the 210 patients analyzed in this study, 83.8% (176 of 210) were homozygous for the A/A variant, 16.2% (34 of 210) were heterozygous for A/G, and none were homozygous for G/G variant. The correlation between the polymorphic variants and the clinicopathologic factors was carefully analyzed (Table 1). Patients with the MMP-7-181A/G genotype were significantly more likely to have lymph node metastasis (P=0.037) and lymphovascular invasion (P=0.003) than those with the A/A genotype. Other variants, including gender, age, tumor size, tumor site, differentiation, Lauren classification, depth of invasion and distant metastasis, had no significant correlation with the MMP-7-181 genotype.
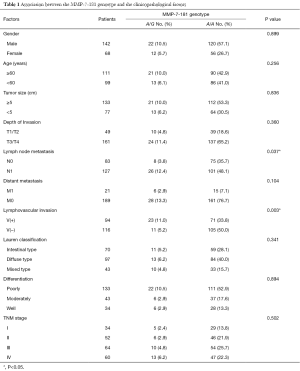
Full table
The association between MMP-7-181 polymorphic variants and prognosis of patients
Univariate analysis detected that 5-year overall survival rates of patients with A/A and A/G genotypes were 59.56% and 33.33%, respectively, and this difference is statistically significant (P=0.016, Figure 2). Patients with the A/G genotype had worse prognoses than those with the A/A genotype. TNM stage and lymphovascular invasion were also significantly correlated with 5-year overall survival, while other variants demonstrated no significant correlation. Multivariate analysis adjusting for sex, age, tumor size, tumor location, depth of invasion, lymph node metastasis, distant metastasis, TNM stages and grade of differentiation revealed that the MMP-7-181A/G genotype (RR =2.098; 95% CI: 1.09–4.05; P=0.027, Table 2) and TNM stage were significant prognostic indicators.
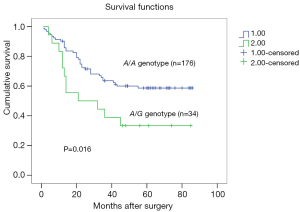
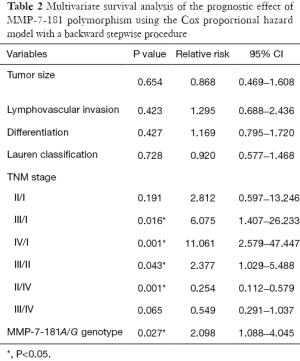
Full table
Discussion
This retrospective study detected the MMP-7-181A/G genotype in gastric cancer and found that there were close correlations between the MMP-7-181A/G genotype and lymph node metastasis and lymphovascular invasion. Univariate and multivariate analyses revealed that the MMP-7-181A/G genotype was a powerful prognosticator for overall survival with gastric cancer patients. Patients with the MMP-7-181A/G genotype are at a higher risk for lymph node metastasis, lymphovascular invasion and poorer prognosis than those with the A/A genotype. Our data strongly suggest that the MMP-7-181A/G genotype may serve as a valuable prognostic indicator for predicting poor prognosis in patients with gastric cancer.
MMPs play a role in the process of cancer invasion and metastasis. MMP-7 is the smallest known member of the MMP family, possessing the highest extracellular matrix (ECM)-degradative activity against a variety of ECM components, including elastin, gelatin, type IV collagen, fibronectin, vitronectin, laminin, entactin, aggrecan and proteoglycans. MMP-7 is overexpressed in invasive cancers of the digestive tract, including esophageal, gastric, colorectal, pancreatic and liver, and its over expression is implicated in cancer transformation, aggressive phenotype, progression, metastasis and poor patient survival (35-39). MMP-7 is most frequently identified in gastric precancerous lesions and gastric carcinomas, but the underlying mechanism of its overexpression has not been fully investigated. Previous studies showed that MMP-7 expression was up-regulated by HP in gastritis and by gastrin in hypergastrinemia and that both of these lesions are associated with gastric carcinoma (40,41). Studies suggested that the functional polymorphisms in the promoters of MMP genes were strongly associated with the risk of multiple types of cancer. Other studies revealed a significant association of functional MMP-7 gene variants toward susceptibility to Hp-induced precancerous gastric lesions (31,32,34,40,41). The MMP-7-181 G allele was found to confer gastric cancer susceptibility and higher transcriptional activity, which correlate with MMP-7 over expression.
The frequencies of each MMP-7-181 genotype differ between studies from different ethnicities and countries. The G allele frequency was shown to be between 8.8% and 42.0%. Moreover, the correlation between the MMP-7-181 polymorphism and individual types of cancer demonstrated certain discrepancies between different studies. Nevertheless, the value of MMP polymorphisms as susceptibility and prognosis indicators is evident and warrants further exploration. The frequency of the G allele in our study is consistent with that of Sugimoto et al. The frequencies of the MMP-7-181A/A, A/G, and G/G genotypes in the gastric cancer group were 83.1%, 16.9%, and 0.0%, respectively in Sugimoto et al.’s study. The previous study also showed that the G allele is associated with an increased risk of gastric cancer (OR =2.31) and that this cancer occurs at a more advanced stage (42). These results are also consistent with our findings, yet Sugimoto et al.’s study demonstrated no significant prognostic value of the MMP-7-181A/G polymorphism. In a study by Kubben et al., the MMP-7-181A/G polymorphism was found to be significantly associated with patient survival (32).
The studies exploring the MMP-7-181A/G genotype commonly used fresh blood samples or normal tissue specimens for DNA extraction, which are adequate methods for studies exploring the susceptibility of cancers. Studies of cancer prognostication may need long-term follow-up information, which is not possible to collect when the original sample consists of fresh blood because DNA quality in fresh blood decreases over time. Archival formalin fixation paraffin-embedded tumor tissue may be possible to as good source for regular, molecular marker detection and exploration of new prognosticators in such retrospective studies. Recent researches have showed that the fixation process does not seem to impact the DNA quality for genotype detection. Genotyping results obtained from paraffin-embedded tissue DNA and peripheral blood DNA are strongly consistent (43). We picked lymph node tissue to detect the genotypes of MMP-7, all the chosen patients had complete pathological examination and follow-up information, and the lymph nodes were histopathologically confirmed with no sign of metastasis. These results provide a good point to further explore the polymorphisms of MMPs in gastric cancer prognostication.
In summary, our results reveal that the MMP-7-181A/G polymorphism is correlated with lymph node metastasis and vascular invasion. The MMP-7-181A/G genotype may serve as a new valuable prognostic indicator in gastric cancer patients.
Acknowledgments
Funding: This work was supported by a Grant for Key Technologies Research and Development Program 2002BA711A06 and the National Basic Research Priorities Program 973 Project 1998051203 from the Ministry of Science and Technology of China, and grant H020920030390 from the Beijing Science and Technology Commission.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted under the assent of the ethics and research committee of Peking University School of Oncology (approval number: V01&2011-3) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Lakhani SR, Ashworth A. Microarray and histopathological analysis of tumours: the future and the past? Nat Rev Cancer 2001;1:151-7. [Crossref] [PubMed]
- Bell CG. Accessing and selecting genetic markers from available resources. Methods Mol Biol 2011;760:1-17. [Crossref] [PubMed]
- Clapper ML. Genetic polymorphism and cancer risk. Curr Oncol Rep 2000;2:251-6. [Crossref] [PubMed]
- Conforti-Froes N, el-Zein R, Au W. Genetic polymorphism and their contribution to cancer susceptibility. Cad Saude Publica 1998;14:7-13. [Crossref] [PubMed]
- Ye S. Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol 2000;19:623-9. [Crossref] [PubMed]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001;17:463-516. [Crossref] [PubMed]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161-74. [Crossref] [PubMed]
- Clark IM, Swingler TE, Sampieri CL, et al. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol 2008;40:1362-78. [Crossref] [PubMed]
- Hinoda Y, Okayama N, Takano N, et al. Association of functional polymorphisms of matrix metalloproteinase (MMP)-1 and MMP-3 genes with colorectal cancer. Int J Cancer 2002;102:526-9. [Crossref] [PubMed]
- Ghilardi G, Biondi ML, Erario M, et al. Colorectal carcinoma susceptibility and metastases are associated with matrix metalloproteinase-7 promoter polymorphisms. Clin Chem 2003;49:1940-2. [Crossref] [PubMed]
- Zhou Y, Yu C, Miao X, et al. Substantial reduction in risk of breast cancer associated with genetic polymorphisms in the promoters of the matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 genes. Carcinogenesis 2004;25:399-404. [Crossref] [PubMed]
- Yu C, Zhou Y, Miao X, et al. Functional haplotypes in the promoter of matrix metalloproteinase-2 predict risk of the occurrence and metastasis of esophageal cancer. Cancer Res 2004;64:7622-8. [Crossref] [PubMed]
- Zhou Y, Yu C, Miao X, et al. Functional haplotypes in the promoter of matrix metalloproteinase-2 and lung cancer susceptibility. Carcinogenesis 2005;26:1117-21. [Crossref] [PubMed]
- Hu Z, Huo X, Lu D, et al. Functional polymorphisms of matrix metalloproteinase-9 are associated with risk of occurrence and metastasis of lung cancer. Clin Cancer Res 2005;11:5433-9. [Crossref] [PubMed]
- Elander N, Söderkvist P, Fransén K. Matrix metalloproteinase (MMP) -1, -2, -3 and -9 promoter polymorphisms in colorectal cancer. Anticancer Res 2006;26:791-5. [PubMed]
- Li Y, Jin X, Kang S, et al. Polymorphisms in the promoter regions of the matrix metalloproteinases-1, -3, -7, and -9 and the risk of epithelial ovarian cancer in China. Gynecol Oncol 2006;101:92-6. [Crossref] [PubMed]
- O-charoenrat P. Leksrisakul P, Sangruchi S. A functional polymorphism in the matrix metalloproteinase-1 gene promoter is associated with susceptibility and aggressiveness of head and neck cancer. Int J Cancer 2006;118:2548-53. [Crossref] [PubMed]
- Lièvre A, Milet J, Carayol J, et al. Genetic polymorphisms of MMP1, MMP3 and MMP7 gene promoter and risk of colorectal adenoma. BMC Cancer 2006;6:270. [Crossref] [PubMed]
- Zhai Y, Qiu W, Dong XJ, et al. Functional polymorphisms in the promoters of MMP-1, MMP-2, MMP-3, MMP-9, MMP-12 and MMP-13 are not associated with hepatocellular carcinoma risk. Gut 2007;56:445-7. [Crossref] [PubMed]
- Xu E, Xia X, Lü B, et al. Association of matrix metalloproteinase-2 and -9 promoter polymorphisms with colorectal cancer in Chinese. Mol Carcinog 2007;46:924-9. [Crossref] [PubMed]
- Tang Y, Zhu J, Chen L, et al. Associations of matrix metalloproteinase-9 protein polymorphisms with lymph node metastasis but not invasion of gastric cancer. Clin Cancer Res 2008;14:2870-7. [Crossref] [PubMed]
- Li Y, Jia JH, Kang S, et al. The functional polymorphisms on promoter region of matrix metalloproteinase-12, -13 genes may alter the risk of epithelial ovarian carcinoma in Chinese. Int J Gynecol Cancer 2009;19:129-33. [Crossref] [PubMed]
- Peng B, Cao L, Wang W, et al. Polymorphisms in the promoter regions of matrix metalloproteinases 1 and 3 and cancer risk: a meta-analysis of 50 case-control studies. Mutagenesis 2010;25:41-8. [Crossref] [PubMed]
- Ayşegül B, Veysi GH, Muzaffer M, et al. Is a single nucleotide polymorphism a risk factor for lung cancer in the matrix metalloproteinase-2 promoter? Mol Biol Rep 2011;38:1469-74. [Crossref] [PubMed]
- Okamoto K, Mandai M, Mimura K, et al. The association of MMP-1, -3 and -9 genotypes with the prognosis of HCV-related hepatocellular carcinoma patients. Res Commun Mol Pathol Pharmacol 2005;117-118:77-89. [PubMed]
- Rollin J, Régina S, Vourc'h P, et al. Influence of MMP-2 and MMP-9 promoter polymorphisms on gene expression and clinical outcome of non-small cell lung cancer. Lung Cancer 2007;56:273-80. [Crossref] [PubMed]
- Beeghly-Fadiel A, Shu XO, Long J, et al. Genetic polymorphisms in the MMP-7 gene and breast cancer survival. Int J Cancer 2009;124:208-14. [Crossref] [PubMed]
- Achyut BR, Ghoshal UC, Moorchung N, et al. Transforming growth factor-B1 and matrix metalloproteinase-7 promoter variants induce risk for Helicobacter pylori-associated gastric precancerous lesions. DNA Cell Biol 2009;28:295-301. [Crossref] [PubMed]
- Zhang J, Jin X, Fang S, et al. The functional polymorphism in the matrix metalloproteinase-7 promoter increases susceptibility to esophageal squamous cell carcinoma, gastric cardiac adenocarcinoma and non-small cell lung carcinoma. Carcinogenesis 2005;26:1748-53. [Crossref] [PubMed]
- Kubben FJ, Sier CF, Meijer MJ, et al. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. Br J Cancer 2006;95:744-51. [Crossref] [PubMed]
- Jormsjö S, Whatling C, Walter DH, et al. Allele-specific regulation of matrix metalloproteinase-7 promoter activity is associated with coronary artery luminal dimensions among hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 2001;21:1834-9. [Crossref] [PubMed]
- Peng B, Cao L, Ma X, et al. Meta-analysis of association between matrix metalloproteinases 2, 7 and 9 promoter polymorphisms and cancer risk. Mutagenesis 2010;25:371-9. [Crossref] [PubMed]
- Honda M, Mori M, Ueo H, et al. Matrix metalloproteinase-7 expression in gastric carcinoma. Gut 1996;39:444-8. [Crossref] [PubMed]
- Liu XP, Kawauchi S, Oga A, et al. Prognostic significance of matrix metalloproteinase-7 (MMP-7) expression at the invasive front in gastric carcinoma. Jpn J Cancer Res 2002;93:291-5. [Crossref] [PubMed]
- Ajisaka H, Yonemura Y, Miwa K. Correlation of lymph node metastases and expression of matrix metalloproteinase-7 in patients with gastric cancer. Hepatogastroenterology 2004;51:900-5. [PubMed]
- Koskensalo S, Mrena J, Wiksten JP, et al. MMP-7 overexpression is an independent prognostic marker in gastric cancer. Tumour Biol 2010;31:149-55. [Crossref] [PubMed]
- Zhang M, Zhu GY, Gao HY, et al. Expression of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in gastric adenocarcinoma. J Surg Oncol 2011;103:243-7. [Crossref] [PubMed]
- Wroblewski LE, Noble PJ, Pagliocca A, et al. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci 2003;116:3017-26. [Crossref] [PubMed]
- Varro A, Kenny S, Hemers E, et al. Increased gastric expression of MMP-7 in hypergastrinemia and significance for epithelial-mesenchymal signaling. Am J Physiol Gastrointest Liver Physiol 2007;292:G1133-40. [Crossref] [PubMed]
- Sugimoto M, Furuta T, Kodaira C, et al. Polymorphisms of matrix metalloproteinase-7 and chymase are associated with susceptibility to and progression of gastric cancer in Japan. J Gastroenterol 2008;43:751-61. [Crossref] [PubMed]
- Rae JM, Cordero KE, Scheys JO, et al. Genotyping for polymorphic drug metabolizing enzymes from paraffin-embedded and immunohistochemically stained tumor samples. Pharmacogenetics 2003;13:501-7. [Crossref] [PubMed]


