
Targeting pancreatic stellate cells to improve pancreatic cancer radiosensitivity
Pancreatic ductal adenocarcinoma (PDAC) is the second most frequent digestive tumour after colorectal cancer, and its incidence is increasing. At present, PDAC is the fourth commonest cause of cancer-related death in developed countries, and it is likely to move up to the second place by 2030 (1). Currently, the 5-year survival rate is quoted at 5–7%, with no significant change over the last 10 years. One of reasons why PDAC remains therapeutically challenging is its inherent resistance to chemotherapy, radiotherapy and immunotherapy (2). Research efforts are being made to understand the biological mechanisms involved in aggressive nature of PDAC and to develop therapies to improve the clinical outcomes.
Over the last few years, researcher attention has been primarily focused on the microenvironment adjacent to tumour cells and its significance to cancer development, progression, and resistance to therapy (3). PDAC compared to other types of cancer is poorly vascularised and displays extensive fibrosis, which is due to the dramatic desmoplastic reaction (3). The desmoplastic stroma is a complex structural environment composed of extracellular matrix (ECM) and a variety of cells including pancreatic stellate cells (PSC), endothelial, and immune cells (3). Activated PSC are thought to be related to cancer-associated fibroblasts (CAF) and are responsible for the ECM protein synthesis in PDAC (mainly, collagen type I and fibronectin) (4). Moreover, PSC secrete numerous factors such as epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), fibroblast growth factor (FGF), connective tissue growth factor (CTGF), and matrix metalloproteinases (MMP), promoting tumour growth, invasion, metastatic potential as well as resistance to chemotherapy and radiotherapy (4).
Recently, Al-Assar et al. reported new preclinical data about the role of PSC in PDAC radioresistance (5). The authors had previously shown in a phase I trial that nelfinavir (NFV) is safe with chemoradiation (CRT) in PDAC and could enhance radiotherapy efficacy (6). Reverse translationally, they aimed to test the influence of PSC on NFV-mediated radiosensitisation to PDAC preclinically. First, they used an in vitro culture model of three different PDAC cell lines (Panc-1, MiaPaCa-2, and PSN-1) with or without human PSC line (hPSC) (direct 2D co-culture), treated by radiation with or without NFV, under normoxic and hypoxic conditions. Of note, information about the ratio PSC: cancer cell was not provided, whilst it would have been of interest since our group and others have shown that this ratio is crucial for the interpretation of the interactions between these two cell types (7). The results revealed heterogeneity between PDAC cell lines regarding the radioprotective effect of PSC. Indeed, a protective effect of hPSC seemed to exist with Panc-1 and PSN-1 cell lines but not with MiaPaCa-2 in clonogenic assays, although no statistical tests were reported. NFV treatment increased the radiosensitivity of the three cell lines, either cultured alone or in co-culture with PSC. The authors assessed the expression levels of pFAK and pAKT, two pathways that have been hypothesized to be modulated by NFV, by Western Blot. They stated that they observed a decrease in FAK and AKT phosphorylation only in the hPSC line. However, careful examination of complete Western Blot data, which are displayed as Suppl. Material, showed a decrease in pFAK in cancer cells lines as well. This observation would be consistent with an effect of NFV both on PDAC cells and PSC, and could account for the fact that the radiosensitisation effect on cancer cells was observed both with and without PSC. They further focused on the Panc-1 and PSN-1 cell lines for the hypoxic/normoxic conditions experiments. The results showed that the radioprotective effect of PSC toward cancer cells seemed unchanged in PSN-1 cells in hypoxic vs. normoxic conditions (surviving fraction of about 20% and 30% without and with hPSC, respectively) while there was an increase in the radioprotective effect of hPSC on Panc-1 cells in normoxic conditions compared with hypoxic conditions: the surviving fraction was 15–20% without hPSC in both normoxic and hypoxic conditions, whereas with hPSC it moved up from 20% in hypoxic conditions to more than 60% in normoxic conditions (no statistical comparison was provided). The authors did not comment on this finding that illustrates again the heterogeneity between PDAC cell lines regarding their interactions with PSC. A marked decrease in the surviving fraction was observed with NFV in normoxic conditions, and to a less extent in hypoxic conditions. Here again, unfortunately, no statistical test result is provided to conclude about the statistical significance of the differences observed. Of note, human PDAC are known to be hypoxic tumours; thus, the effect of NFV in the clinical setting would be expected to be closer to the hypoxic than the normoxic model, and less dramatic than the normoxic in vitro model would suggest.
Then, the authors assessed the effect of radiation +/− NFV on tumour growth in vivo in a mouse model of subcutaneous xenograft of PSN-1 cells with or without hPSC. Their results showed that: (I) NFV alone had no effect on tumour growth; (II) radiotherapy decreased tumour growth only in the absence of PSC; (III) the effect of radiotherapy in this context was increased by the addition of NFV; (IV) in the presence of PSC, the addition of NFV to radiation slowed tumour growth so that the growth curve tends to be close to the one of tumours without PSC treated with radiation only, suggesting that NFV could reverse the protective effect of PSC. Nonetheless, the small number of animals per group (n=4 or 5) and the large confidence intervals do not allow us to draw definitive conclusion. Noticeably, subcutaneous xenograft models are more vascularized, thus less hypoxic, tumours than orthotopic models and this may have yielded to an overestimation of the magnitude of the effect. Moreover, toxicity data in the mice are not presented. Overall, the Al-Assar et al. article displays notable weaknesses: data were only partially presented, important information was missing, interpretation of the results was debatable, some of the models were questionable, and statistical analyses were not available. In our view, the main conclusions from the authors’ findings are quite different from the data they chose to highlight, and would be that: (I) PDAC cells are heterogeneous regarding PSC radioprotection; and (II) NFV exerts radioprotective effects that may be mediated by FAK pathway inhibition in both PDAC and PSC cells. These findings would be consistent with other recent works highlighting the emerging role of FAK as a therapeutic target both in cancer and stromal PDAC cells (8). It remains unclear what happened in the xenografts formed by cancer cells plus hPSC and treated by NFV and radiation; indeed, the histological analysis of these tumours was not provided. The hypothesis of the authors is that NFV targets PSC leading to a breakdown in the PSC-cancer cell interactions. Given the data by Rhim et al. (9) and Özdemir et al. (10) that showed that the elimination of PSC from PDAC stroma is deleterious, leading to unfavourable immune microenvironment modulation and tumour de-differentiation, it would be relevant to ask whether NFV results in a decrease in PSC viability or reprograms them toward a non-activated phenotype.
Over the last 10 years, NFV has emerged as an interesting radiosensitising agent in the management of cancer (11). NFV is originally an antiretroviral drug commonly used in the treatment of human immunodeficiency virus (HIV) infection. NFV is a protease inhibitor designed to target HIV-1 and HIV-2 proteases, which are important for the replication and release of the virus. Following the observation of anti-tumour activity on Kaposi sarcoma in HIV patients, preclinical data have accumulated for an effect of HIV protease inhibitors (HPI) on immune system reconstitution as well as by targeting tumour cell signalling pathways, paving the way for clinical trials in non-HIV patients (12). HPI have been found to be an effective addition agent when used alongside radiotherapy (11). Most of radiosensitising agents work by targeting tumour hypoxia to improve sensitivity to radiotherapy, either by increasing oxygen delivery or by altering tumour oxygen consumption (11). Hence, HPI agents have been shown to have an off-target effect on the PI3K-AKT-mTOR pathway leading to downregulation of mTOR pathway, resulting in altered metabolism, reduced tumour growth and enhanced tumour cell sensitivity to radiation both in vitro and in vivo. NFV is hypothesized to decrease AKT phosphorylation indirectly by inhibition of proteasome thereby triggering an unfolded protein response (13). NFV is thought to exert its radiosensitising effect mainly through this mechanism of modulation of tumour oxygen consumption (11). However, clinical evidence also demonstrated that tumour perfusion was increased following treatment with NFV; thus, either of the mechanisms could be involved (14).
NFV has been tested in the clinical setting mainly in rectal, head and neck, lung, and pancreatic cancer, either alone or in combination with radiation therapy (6,14-21). This drug has been evaluated only in phase Ib to II studies, and has not reached phase III study to date. Data from these studies are summarised in Table 1 and ongoing trials are presented in Table 2. Overall, NFV failed to demonstrate sufficient activity when used as monotherapy, but positive signals for phase II development were reported in combination with radiotherapy.
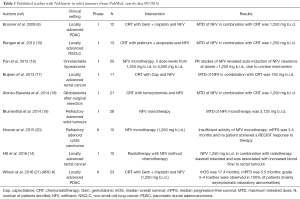
Full table
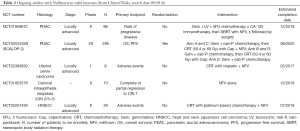
Full table
As far as PDAC is concerned, radiation therapy is a modality for treatment of locally advanced and resectable PDAC. In both settings, radiotherapy has been a matter of debate for many years. In the situation of resectable PDAC, the use of adjuvant radiotherapy is regarded as a controversial topic with inconsistent results. European studies have been unable to identify a benefit whereas North American groups encourage the use of this therapeutic modality for local control (22-25). The Pancreatic Cancer Meta-Analysis Group publication showed a benefit of adjuvant chemoradiotherapy (CRT) for the subgroup of patients whose resection was incomplete (R1 resection) (26). CRT is thus considered as an alternative to adjuvant chemotherapy in resected PDAC cases with positive margins (R1 or R2 resection), as well as for patients with positive lymph nodes. The RTOG-0848 randomised trial, which aims to answer the question of CRT in the adjuvant setting, is ongoing (NCT01013649). In addition, multiple studies are currently in progress addressing the possible use of radiotherapy in the neoadjuvant setting for borderline resectable tumours, in combination with chemotherapy (2).
In the circumstance of locally advanced PDAC (LAPC), two randomised prospective studies comparing front-line CRT vs. gemcitabine alone showed contradictory results, while two other retrospective studies were in favour of the use of CRT after a course of chemotherapy (27-30). The randomised LAP07 and SCALOP studies prospectively tested this second strategy and recently provided key results about radiation therapy in LAPC. In the SCALOP phase II study, LAPC patients with stable or responding disease after induction chemotherapy by three cycles of gemcitabine plus capecitabine (GEMCAP) combination further received radiation therapy associated with either capecitabine or gemcitabine (31). The capecitabine arm was significantly superior in term of median overall survival (OS) (15.2 vs. 13.4 months; HR: 0.39; P=0.012), with less haematological and non-haematological side effects compared to the gemcitabine arm. CRT with capecitabine should then form the template regimen for radiation therapy in LAPC. On the other hand, the LAP07 phase III study used a two-randomisation study plan with gemcitabine with or without erlotinib as induction chemotherapy (32). If patient’s tumours were controlled after four months of treatment, they were randomised for a second time to continuation of the same chemotherapy or CRT with capecitabine. Erlotinib addition did not improve OS, and no significant survival difference was observed between the chemotherapy and CRT groups. A secondary analysis showed that patients in the CRT group had a longer treatment-free period with significantly less local tumour progression. Current CONKO-007 (NCT01827553) phase III trial and LAPACT (NCT02301143) randomised phase II trial are being conducted to assess the role of CRT with the prospect of more active induction chemotherapy regimens, i.e., the FOLFIRINOX and gemcitabine plus nab-paclitaxel combinations, respectively, which have shown superiority over single-agent gemcitabine in metastatic PDAC setting. Thus radiotherapy alongside chemotherapy is being still explored in LAPC in an unselected manner as possible means to extend survival.
NFV has been evaluated in LAPC in the ARCII phase II trial (21). Radiotherapy was administered concomitantly with weekly gemcitabine and cisplatin. NFV was administered orally from 3–10 days before CRT start and was continued during CRT. The primary end-point was 1-year OS. The study closed prematurely after recruiting 23 patients due to non-availability of NFV in Europe. Combination of NFV with CRT demonstrated encouraging efficacy effects (median OS 17.4 months) at the expense of high toxicity with 100% of patients experiencing grade 3–4 adverse events. This high level of toxicity is thought to be partially related to the underlining gemcitabine-based chemotherapy regimen, and it is expected that a capecitabine-based CRT may have a more favourable profile. The SCALOP-2 phase II trial (NCT02024009), is an ongoing multi-centre randomised study where patients with LAPC will be treated with induction gemcitabine plus nab-paclitaxel followed by random assignment to further chemotherapy or to CRT with capecitabine with or without NFV (NCT02024009). The estimated date of completion is August 2020.
Based on the same rationale as NFV, other agents targeting PSC may be relevant to use in combination with CRT. Our group has explored stromal targeting in PDAC with PSC modulating agents to enhance the anti-tumour effect of chemotherapy (33,34). We have showed that all trans retinoic acid (ATRA) was able to induce PSC quiescence, leading to reduced proliferation and increased apoptosis of surrounding pancreatic cancer cells (33). Using organotypic co-culture and mouse models, we demonstrate a reduction in cancer cell proliferation and invasion together with enhanced cancer cell apoptosis when ATRA was combined with gemcitabine, compared to vehicle or either agent alone (34). These effects were mediated through a range of signalling cascades (Wnt, hedgehog, retinoid, and FGF) in cancer as well as PSC, affecting epithelial cellular functions such as epithelial-mesenchymal transition, cellular polarity, and lumen formation. Remarkably, at the tissue level, ATRA treatment enhanced tumour necrosis, increased tumour vascularity and reduced hypoxia in the tumour microenvironment (34). Such changes would be hypothesized to sensitise tumours to CRT. We are currently conducting a phase I study to evaluate ATRA in combination with gemcitabine plus nab-paclitaxel chemotherapy in patients with advanced PDAC (STARPAC trial, EudraCT2015-002662-23).
Finally, by highlighting the heterogeneity in the effects of PSC on cancer cells in the context of radiotherapy, Al-Assar article implies the importance of patient selection and identification of predictive biomarkers of response. It is not clear based on Al-Assar data which molecular characteristics of the cell lines make them more prone to respond to NFV and radiation therapy combination. Alternatively, molecular markers such as SMAD4 and TP53 may help identify patients whom are at low prospective of developing distant metastasis and thus, are more likely to benefit from CRT (35).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.68). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Neuzillet C, Tijeras-Raballand A, Bourget P, et al. State of the art and future directions of pancreatic ductal adenocarcinoma therapy. Pharmacol Ther 2015;155:80-104. [Crossref] [PubMed]
- Neesse A, Algül H, Tuveson DA, et al. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 2015;64:1476-84. [Crossref] [PubMed]
- Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 2013;144:1210-9. [Crossref] [PubMed]
- Al-Assar O, Bittner MI, Lunardi S, et al. The radiosensitizing effects of Nelfinavir on pancreatic cancer with and without pancreatic stellate cells. Radiother Oncol 2016;119:300-5. [Crossref] [PubMed]
- Brunner TB, Geiger M, Grabenbauer GG, et al. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol 2008;26:2699-706. [Crossref] [PubMed]
- Kadaba R, Birke H, Wang J, et al. Imbalance of desmoplastic stromal cell numbers drives aggressive cancer processes. J Pathol 2013;230:107-17. [Crossref] [PubMed]
- Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 2016;22:851-60. [Crossref] [PubMed]
- Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014;25:735-47. [Crossref] [PubMed]
- Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719-34. [Crossref] [PubMed]
- Lin A, Maity A. Molecular Pathways: A Novel Approach to Targeting Hypoxia and Improving Radiotherapy Efficacy via Reduction in Oxygen Demand. Clin Cancer Res 2015;21:1995-2000. [Crossref] [PubMed]
- Sgadari C, Monini P, Barillari G, et al. Use of HIV protease inhibitors to block Kaposi's sarcoma and tumour growth. Lancet Oncol 2003;4:537-47. [Crossref] [PubMed]
- Gupta AK, Li B, Cerniglia GJ, et al. The HIV protease inhibitor nelfinavir downregulates Akt phosphorylation by inhibiting proteasomal activity and inducing the unfolded protein response. Neoplasia 2007;9:271-8. [Crossref] [PubMed]
- Hill EJ, Roberts C, Franklin JM, et al. Clinical Trial of Oral Nelfinavir before and during Radiation Therapy for Advanced Rectal Cancer. Clin Cancer Res 2016;22:1922-31. [Crossref] [PubMed]
- Rengan R, Mick R, Pryma D, et al. A phase I trial of the HIV protease inhibitor nelfinavir with concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non-small cell lung cancer: a report of toxicities and clinical response. J Thorac Oncol 2012;7:709-15. [Crossref] [PubMed]
- Pan J, Mott M, Xi B, et al. Phase I study of nelfinavir in liposarcoma. Cancer Chemother Pharmacol 2012;70:791-9. [Crossref] [PubMed]
- Buijsen J, Lammering G, Jansen RL, et al. Phase I trial of the combination of the Akt inhibitor nelfinavir and chemoradiation for locally advanced rectal cancer. Radiother Oncol 2013;107:184-8. [Crossref] [PubMed]
- Alonso-Basanta M, Fang P, Maity A, et al. A phase I study of nelfinavir concurrent with temozolomide and radiotherapy in patients with glioblastoma multiforme. J Neurooncol 2014;116:365-72. [Crossref] [PubMed]
- Blumenthal GM, Gills JJ, Ballas MS, et al. A phase I trial of the HIV protease inhibitor nelfinavir in adults with solid tumors. Oncotarget 2014;5:8161-72. [Crossref] [PubMed]
- Hoover AC, Milhem MM, Anderson CM, et al. Efficacy of nelfinavir as monotherapy in refractory adenoid cystic carcinoma: Results of a phase II clinical trial. Head Neck 2015;37:722-6. [Crossref] [PubMed]
- Wilson JM, Fokas E, Dutton SJ, et al. ARCII: A phase II trial of the HIV protease inhibitor Nelfinavir in combination with chemoradiation for locally advanced inoperable pancreatic cancer. Radiother Oncol 2016;119:306-11. [Crossref] [PubMed]
- Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005). J Clin Oncol 2008;26:3511-6. [Crossref] [PubMed]
- Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol 2010;17:981-90. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Stocken DD, Büchler MW, Dervenis C, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer 2005;92:1372-81. [Crossref] [PubMed]
- Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol 2008;19:1592-9. [Crossref] [PubMed]
- Loehrer PJ Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 2011;29:4105-12. [Crossref] [PubMed]
- Huguet F, André T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326-31. [Crossref] [PubMed]
- Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer 2007;110:47-55. [Crossref] [PubMed]
- Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol 2013;14:317-26. [Crossref] [PubMed]
- Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016;315:1844-53. [Crossref] [PubMed]
- Froeling FE, Feig C, Chelala C, et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-ß-catenin signaling to slow tumor progression. Gastroenterology 2011;141:1486-97, 1497.e1-14.
- Carapuça EF, Gemenetzidis E, Feig C, et al. Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma. J Pathol 2016;239:286-96. [Crossref] [PubMed]
- Iacobuzio-Donahue C. Dynamics and evolution of pancreatic cancer from inception to invasion. AACR Special Conference on Pancreatic Cancer: Innovations in Research and Treatment, 2014:abstr IA1.


