
Axon guidance repulsant SEMA3F increases chemosensitivity to oxaliplatin and inhibits epithelial-mesenchymal transition of colorectal cancer cells
Introduction
Axon guidance repulsive Semaphorin-3F (SEMA3F) belongs to the class 3 Semaphorin family, which is a large group of secreted axon guidance molecules involved in the development of nervous system (1). Recent studies have revealed that SEMA3F was widely expressed outside the nervous system and played major roles in the tumor context such as angiogenesis, tumor growth and metastasis (2,3). The SEMA3F gene locates in 3p21.3, a region that is frequently lost in lung cancer and breast cancer, implying that the SEMA3F gene a candidate tumor suppressor gene (4,5). In addition to gene deletion, SEMA3F has been shown to be down-regulated in many kinds of cancers such as lung (4,6), breast (7), ovarian (8), colorectal cancer (CRC) (9), and melanoma (10). Functionally, SEMA3F has been reported to inhibit proliferation, invasion, adhesion of cancer cells (1). However, the role of SEMA3F in chemosensitivity of cancer cells remains elusive.
CRC is the third most common cancer and the fourth leading cause of tumor associated death in the world (11). The incidence of CRC is still increasing in Asia, especially in China (12). Despite numerous efforts have been put into the development of diagnosis and treatment of CRC, the 5-year survival rate of CRC patients remains about 50%, with the five-year survival rate of patients with distant metastasis as low as 10–20% (11). Tumor metastasis is a sophisticated multi-step process, including escape from the primary tumor, intravasation into the lymphatic or vascular systems, survival in the circulation, avoidance of host defense mechanisms, arrest at a new site, extravasation into the tissue, and growth at the new site (13). Since the introduction of oxaliplatin for the treatment of metastatic CRC, improved objective response rate, increased percentage of metastasis resection and prolonged survival have been shown in 40–50% of the CRC patients (14). However, the remaining patients could not benefit from oxaliplatin because of side effects and the drug resistance. Oxaliplatin exerts its cytotoxic effect mainly by inducing DNA damage, which is followed by activation of apoptosis pathways (15). The molecular mechanisms underlying oxaliplatin resistance includes intracellular transport and detoxification, altered DNA damage repair and cell death mechanisms (15). The epithelial-mesenchymal transition (EMT) refers to the program by which epithelial cells shed their differentiated features and acquire mesenchymal characteristics such as motility, invasiveness and a heightened resistance to apoptosis (16). The EMT has been implicated in two of the most important processes responsible for cancer-related mortality: distant metastasis and therapeutic resistance. Remarkably, two recent studies have demonstrated that the EMT process attenuated chemosensitivity to chemotherapy drugs rather than metastasis of breast and pancreatic cancer (17,18).
Our previous study has demonstrated that SEMA3F is down-regulated in CRC samples and functions as an inhibitor of growth (19), metastasis (9) and stemness (20) of colorectal cancer cells, but the role of SEMA3F in EMT and drug resistance to oxaliplatin was poorly known. The present work found that the expression of SEMA3F is negatively correlated with the expression of permeability glycoprotein (P-gp) and glutathione-s-transferase-π (GST-π) in CRC tissues from 94 patients. Down-regulation of SEMA3F by lentivirus particles expressing shRNA against SEMA3F significantly decreased apoptosis and enhanced invasion capacity of CRC HCT116 and SW480 cells. Moreover, decreased epithelial marker E-cadherin expression, and increased mesenchymal marker Slug, Snail and Vimentin expression were found in SEMA3F down-regulating cells. The IC50 to oxaliplatin of SEMA3F down-regulating cells was dramatically higher than that of control cells. In vivo, we showed that down-regulation of SEMA3F increased the tumor volume and decreased overall survival of nude mice using orthotopic CRC xenograft model, even under the treatment with oxaliplatin. Taken together, these results demonstrated that SEMA3F increases chemosensitivity to oxaliplatin and inhibits EMT of CRC cells.
Methods
Cell lines and patient samples
Ninety-four cases of tumor tissues were from colorectal cancer patients who underwent colectomy with lymph node dissection in the period from 2006 to 2008 at Southwest Hospital of Third Military Medical University. The patients received neither chemotherapy nor radiotherapy before surgery. The diagnoses of colorectal carcinoma were made independently by at least 2 histopathologists. This study was carried out in according to the principles of the Helsinki Declaration and approved by the Ethical Committee of the Third Military Medical University (No. 2013062). Written informed consent was obtained from all patients.
The human CRC cell line HCT116, SW480, SW620, HT29 and LoVo were obtained from the American Type Culture Collection. The ATCC catalogs of the cells used in the present study are as follows: SW480, ATCC® CCL-228™; SW620, ATCC® CCL-227™; HCT116, ATCC® CCL-247™; HT29, ATCC® HTB-38™; LoVo, ATCC® CCL-229™. The cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA), 2 U/mL penicillin-streptomycin, vitamins, 1 mmol/L sodium pyruvate, 2 mmol/LL-glutamine, and nonessential amino acids (Thermo Scientific, Watertown, MA, USA) at 37 °C in 5% CO2.
Immunohistochemical staining
Following deparaffinized and rehydrated, tumor sections of 4 µm were incubated in 0.3% H2O2 in methanol for 30 minutes at 37 °C to block endogenous peroxidase. The sections were then boiled in 10 mmol/L citrate buffer (pH =6.0) for 15 minutes in a microwave oven. The antibodies against SEMA3F (Millipore, Billerica, MA, USA), P-gp (MXB Biotechnologies, China), GST-π (MXB Biotechnologies, China) or Topo-II (MXB Biotechnologies, China) was added and the sections were incubated at 4 °C overnight. The sections were visualized using the diaminobenzidine solution (DAKO), and then lightly counterstained with hematoxylin. Sections without incubation with primary antibody served as negative controls. The intensity of staining (brown color) was semi-quantitatively scored as follows: 1, weak; 2, medium; 3, strong; and 4, very strong. The percentage of maximally stained tumor cells in each section was recorded (0, <5%; 1, 5–30%; 2, 30–50%; 3, >50%). High expression of SEMA3F was defined as a combined score for the intensity and area of staining that was ≥2. The results were verified by two histopathologists independently.
Knock-down of SEMA3F
Lentivirus particles expressing short hairpin RNA (shRNA) against SEMA3F, or control shRNA were produced by Neuronbiotech (Shanghai, China) using the sequence we previously used (19). Stable transfectant cells expressing enhanced GFP were isolated with a flow cytometric sorting system (BD FACS Aria II, Bedford, MA, USA). HCT116 cells transfected with shRNA-SEMA3F were named Lv-shSEMA3F, and the cells transfected with scramble sequence were named Lv-control, cells without transfection were named WT.
Quantitative reverse transcriptase-PCR (qRT-PCR)
Total RNA was extracted from cultured cells with Trizol Reagent (TaKaRa, Dalian, China). The concentration and integrity of extracted RNA was examined by UV spectrophotometer. The High Fidelity RNA PCR Kit and SYBR Primescript RT-PCR Kit were purchased from TaKaRa Biotech (Dalian, China). Primers for quantitative PCR were designed using Primer Premier 5.0 software according to the sequence data in the GeneBank. The primers used are as following: SEMA3F (forward: CGAGTGTGGGAACTTCGTCA, reverse: CACATAGGTGCACATGGGGT) and GAPDH (forward: CTATAAATTGAGCCCGCAGCC, reverse: GCGCCCAATACGACCAAATC). The results of three independent experiments were analyzed using the ΔΔC[t] method (21).
Western blot
Total proteins of the cultured cells were extracted with RIPA lysis buffer (Beyotime, Shanghai, China) supplemented with inhibitors of protease and phosphatase. Proteins were then denatured and applied to electrophoresis with SDS-PAGE, followed by being transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Following incubation with 5% nonfat milk in PBST for 2 h at room temperature, the membranes were incubated with primary antibody against SEMA3F (Millipore, Billerica, MA, USA), BCL-2, BAX, BAD, p53, E-cadherin, Vimentin, Snail, Slug or GAPDH antibody (Cell Signaling, Beverly, MA, USA) overnight at 4 °C. The protein abundance was revealed by using HRP-conjugated secondary antibodies (Beyotime, Shanghai, China) and visualized with an enhanced chemiluminescence (ECL) detection system (Thermo Scientific, Watertown, MA, USA).
Immunofluorescence and confocal laser scanning microscopy
Cells transfected with lentivirus were grown on poly-L-Lysine-coated glass coverslips and then fixed for 20 minutes in 4% paraformaldehyde at room temperature. The cells were then rinsed 3 times with PBS for 5 minutes each time, followed by incubating in a protein-blocking solution for 30 minutes at 37 °C After incubation with the primary antibody against human SEMA3F (1:100; Millipore, Billerica, MA, USA) overnight at 4 °C and being rinsed with PBS, the cells were incubated with the secondary antibody (TRITC-conjugated IgG, 1:100, Beyotime, Shanghai, China) at 37 °C for 30 minutes and then stained with DAPI (Beyotime, Shanghai, China). The slides were then analyzed by confocal laser scanning microscopy.
Enzyme-linked immunosorbent assay (ELISA)
Lentivirus transfected cells plated in 6-wells plates were cultured in fresh culture medium for 24 h. The supernatant was then collected after 24 h and the concentration of SEMA3F was measured using ELISA kits (MyBioSource, San Diego, CA, USA) as previously described (22). The results were presented as the concentration of SEMA3F per 106 cells.
Apoptosis assay
Cells seeded in 6-wells plates were incubated in fresh culture medium supplemented with or without 10 µM oxaliplatin for 12 h. The cells were then collected with accutase (Thermo Scientific, Watertown, MA, USA) and stained with AnnexinV-APC and PI (eBioscience, San Diego, CA, USA) according to the manufacture’s instructions. The cells were then analysed using a flow cytometric sorting system (BD FACS Aria II, Bedford, MA, USA).
Cell viability assay
To test the proliferation of Lv-shSEMA3F and Lv-control cells, five hundred cells were seeded in 96-wells plates. The cells were then incubated with the CCK-8 (Beyotime, Shanghai, China) for 4 h at the indicated time points. Cell viability was also determined by CCK-8 kit (Beyotime, Shanghai, China). One thousand cells were seeded and cultured in 96-wells plates. After overnight recovery, the cells were incubated in fresh culture medium containing different concentration of oxaliplatin (Sigma-Aldrich, St. Louis, MO, USA) for 72 h. The half-maximal inhibitory concentration (IC50) was calculated by the Graph Pad software.
Cell invasion assays
The invasion capacity of HCT116 and SW480 cells was evaluated in transwells (BD Falcon, Franklin Lakes, NJ, USA). The cells (5×104/well) in serum-free medium were seeded into MatrigelTM (BD Biosciences, Franklin Lakes, NJ)-coated upper chambers. After incubation for 36 hours at 37 °C, the remaining cells left in the upper chamber were removed and the cells in the lower chambers were fixed and stained with crystal violet. The number of cells was counted in 5 distinct areas at ×400 magnification. The results represent the average cell number in 3 wells per cell line.
Orthotopic xenografts
Four-week-old male nude mice were caged in groups of 5 and acclimated for a week. Animal care was provided according to the Guidelines for the Care and Use of Laboratory Animals. To establish orthotopic xenografts, the mice were anesthetized with chloral hydrate and subjected to laparotomy (n=40). Afterwards, 5×106 cells (Lv-shSEMA3F or Lv-control) in 20 µL Matrigel containing growth factors (BD Biosciences, Franklin Lakes, NJ, USA) were injected into the subserosa of the mouse colon. Three weeks after implantation, mice were divided into four treatment groups (n=10 per group). Group one were injected with Lv-shSEMA3F cells and received PBS, group two were injected with Lv-shSEMA3F cells and received oxaliplatin 6 mg/kg/week i.p, group three were injected with Lv-control cells and received PBS, group four were injected with Lv-control cells and received oxaliplatin 6 mg/kg/week i.p. Xenografts were harvested and fixed in 10% formalin until the mouse died. The tumor volume was calculated using the following equation: Length × width2 × 0.52.
Statistical analysis
All experiments were conducted at least three times and the results were from representative experiments. Data were expressed as mean values ± standard deviation (SD), and the statistical significance between testing and control groups was analysed with SPSS18.0 statistical software. Chi-square analysis was used to analyze the relationship between the expression of SEMA3F and drug resistance-related molecules. When two groups were compared, the unpaired Student’s t-test was used. The Kaplan-Meier method was used to analyze overall survival of mice. P value less than 0.05 was considered statistically significant.
Results
Negative correlation between the expression of SEMA3F and the expression of P-gp and GST-π in CRC tissues
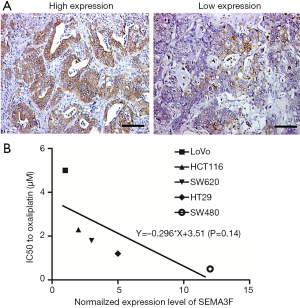
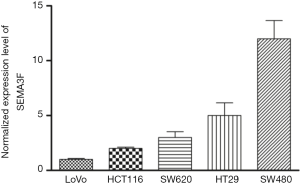
We first examined SEMA3F expression in the specimens from 94 CRC patients, and found that the tumors from 53.2% (50/94) of CRC patients had low SEMA3F expression (Figure 1A). The correlation between SEMA3F expression levels and the expression of P-gp, GST-π or topoisomerase II (Topo-II) are summarized in Table 1. We found that the expression of SEMA3F is negatively correlated with the expression of P-gp and GST-π, but not Topo-II in CRC tissues from 94 patients. Moreover, the expression level of SEMA3F was determined by RT-PCR (Figure S1), which was correlated with the chemosensitivity to oxaliplatin of these CRC cell lines though without significant difference (Figure 1B). These results indicate that SEMA3F is involved in the drug resistance of CRC cells.

Full table
Knock-down of SEMA3F in HCT116 cells by lentivirus transfection
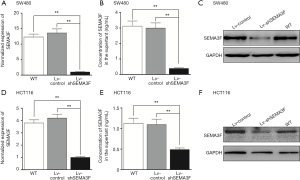
The expression of SEMA3F in CRC cell lines was determined by RT-PCR, showing the expression level of SEMA3F is highest in SW480 cells and lowest in LoVo cells. (Figure S1) The expression level of SEMA3F in HCT116 cells is moderate. HCT116 and SW480 cells were transfected with lentivirus particle expressing shRNA against SEMA3F or scramble sequence. Knock-down of SEMA3F was confirmed by RT-PCR, Western blot and ELISA in SW480 cells (Figure 2A,B,C) and HCT116 cells (Figure 2D,E,F). There was no significant difference between the Lv-control cells and WT cells in the expression or secretion of SEMA3F. On the other hand, the expression or secretion of SEMA3F was significantly reduced in Lv-shSEMA3F cells. These results confirm knock-down of SEMA3F in HCT116 and SW480 cells.
Reduced apoptosis percentage of SEMA3F knock-down cells
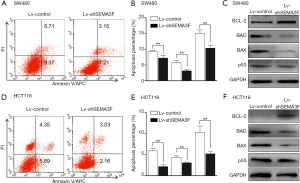
We first test the apoptosis percentage of Lv-shSEMA3F cells and Lv-control cells by AnnexinV-APC and PI staining. The result showed that knock-down of SEMA3F leads to reduced apoptosis percentage in SW480 cells (Figure 3A,B). Expression of apoptosis related proteins was tested by Western blot. The results showed that the anti-apoptotic BCL-2 was up-regulated and the pro-apoptotic BAD and BAX were down-regulated in Lv-shSEMA3F cells compared with Lv-control cells (Figure 3C). However, there was no difference in the expression of tumor suppressor P53. Accordingly, knock-down of SEMA3F leads to reduced apoptosis percentage in HCT116 cells (Figure 3D,E), accompanied with up-regulation of BCL-2 and down-regulation of BAD and BAX (Figure 3F). These results demonstrate that knockdown of SEMA3F reduces apoptosis of CRC cells.
EMT features of SEMA3F knock-down cells
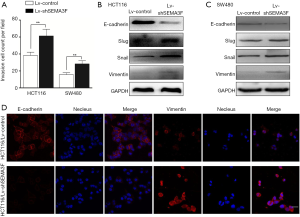
Cell invasion assay showed that knock-down of SEMA3F significantly enhanced invasion capacity of HCT116 and SW480 cells (Figure 4A). As EMT has been recognized as an important process in distant metastasis and therapeutic resistance, the expression of EMT markers was tested by Western blot (Figure 4B,C) and Immunofluorescence (Figure 4D). The results showed that the epithelial marker E-cadherin was down-regulated, while the mesenchymal markers Slug, Snail and Vimentin were up-regulated in Lv-shSEMA3F cells in comparison with Lv-control cells. These results reveal that knock-down of SEMA3F promotes EMT of CRC cells.
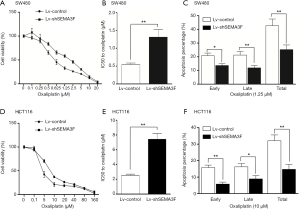
Attenuated chemosensitivity to oxaliplatin of SEMA3F knock-down cells
Oxaliplatin was the first platinum drug with proven anti-CRC activity and has become a standard in the management of CRC. The existence of intrinsic or acquired drug resistance is a major reason for treatment failure (15). The role of SEMA3F in chemosensitivity to oxaliplatin of CRC cells was evaluated by cell viability assay. The result showed that the IC50 to oxaliplatin of Lv-shSEMA3F cells was dramatically higher than control cells. The IC50 to oxaliplatin of SW480 was increased from 0.54 to 1.31 µM following knock-down of SEMA3F (Figure 5A,B; P<0.01). Knock-down of SEMA3F significantly reduced oxaliplatin-induced apoptosis (Figure 5C). In HCT116 cells, the IC50 to oxaliplatin was in 2.54 to 7.43 µM following knock-down of SEMA3F (Figure 5D,E; P<0.01). In addition, knock-down of SEMA3F significantly reduced oxaliplatin-induced apoptosis in HCT116 cells (Figure 5F). In summary, these results demonstrate that knock-down of SEMA3F decreases chemosensitivity to oxaliplatin of CRC cells.
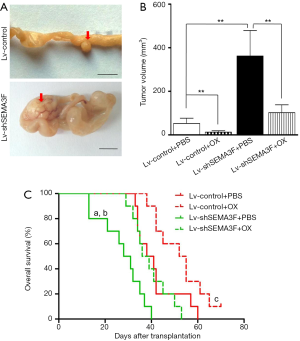
Decreased therapeutic effect of oxaliplatin on SEMA3F knockdown cells in vivo
We finally test the role of SEMA3F in the management of CRC with oxaliplatin in vivo using orthotopic xenograft model. Three weeks after transplantation, mice were treated with oxaliplatin 6 mg/kg/week i.p. or PBS. The volume of tumors originated from Lv-shSEMA3F cells was larger than that from Lv-control cells (Figure 6A,B). Although treatment with oxaliplatin reduced the tumor volume significantly, the tumor volume of Lv-shSEMA3F cells was still larger than that of Lv-control cells. Moreover, the overall survival time of mice transplanted with Lv-shSEMA3F cells was shorter than that of those with Lv-control cells. Under treatment with oxaliplatin, the overall survival time of mice transplanted with Lv-shSEMA3F cells was still shorter (Figure 6C). Taken together, these results revealed that knock-down of SEMA3F attenuates therapeutic effect of oxaliplatin in vivo.
Discussion
Although originally recognized as axon guidance repulsive, SEMA3F has been shown to inhibit the progression of tumor. Bielenberg reported that SEMA3F blocked metastases of melanoma cells transplanted subcutaneously into nude mice and those tumors were poorly vascularized (10). Over-expression of SEMA3F significantly prolonged survival of nude rats orthotopically transplanted with lung cancer cells, accompanied with loss of activated αvβ3 integrins (23). Further study showed that SEMA3F affects multiple signaling pathways in lung cancer cells, including reducing AKT and STAT phosphorylation and integrin-linked kinase activity (24). SEMA3F also had a repulsive effect on motile breast cancer cell line C100 (25). In addition, SEMA3F has been reported to reduce colony formation, adhesion and invasion of ovarian cancer cells, which was associated with reduced phosphorylation of focal adhesion kinase and down-regulation of matrix metalloproteinase-2/9 (26). Our previous work found that down-regulation of SEMA3F was related to short overall survival time and increased distant metastasis (9). More recently, SEMA3F has been reported to inhibit proliferation and metastasis of ileal neuroendocrine tumor cells at least partially through inactivating mTOR and MAPK pathways (27).
The present work found that the expression of SEMA3F is negatively correlated with the expression of P-gp and GST-π in CRC tissues from 94 patients. Both P-gp and GST-π are involved in the chemoresistance of cancer cells. P-gp, also called ABCB1 or MDR1, is a member of the ABC transporter family, which pumps chemotherapeutic agents out of cancer cells (28). GST-π belongs to the Glutathione S-transferases family, which catalyze the conjugation of toxic and carcinogenic electrophilic molecules with GSH to protect macromolecules from damage (29). High expression of GST-π has been reported in colon cancer and in drug-resistant tumors (15). We for the first time report that knock-down of SEMA3F reduces apoptosis and chemosensitivity to oxaliplatin of CRC cells in vitro. Using orthotopic xenograft model, we found that knock-down of SEMA3F attenuated therapeutic effect of oxaliplatin in vivo. In consistence with previous studies, we found that knock-down of SEMA3F increased the vascular density in orthotopic xenografts (data not shown), which may decrease the delivery of oxaliplatin and thus influence the efficiency of oxaliplatin. Moreover, it has been reported that SEMA3F can to bind the Neuropilin-2 receptor (30). The direct effect of VEGF on tumor cells remains poorly known until Bhattacharya et al. reported that the intracellular but not the extracellular VEGF elevated cell viability of CRC cells by activating PI3K/mTOR-AKT signaling (31). As a secreted molecule, SEMA3F activates down-stream signaling through binding to the cell membrane receptors, including Neuropilin-2 and PlexinA1 (1). Therefore, competing with VEGF for binding Neuropilin-2 could not contribute to the pro-apoptosis effect of SEMA3F. On the other hand, the PI3K-AKT signaling, which can be inhibited by SEMA3F, plays fundamental role in survival, growth, invasion and metastasis of cancer cells (32). We therefore propose that SEMA3F exerts its pro-apoptosis effect through inhibiting PI3K-AKT signaling.
Oxaliplatin is the third-generation platinum drug that is used for the treatment of CRC, gastric and pancreatic cancers. Since its introduction in the treatment of metastatic CRC in 2000, oxaliplatin has largely increased objective response rates, percentage of metastasis resection and overall survival of patients (15). Unfortunately, treatment failure occurs mainly due to intrinsic or acquired resistance. The molecular mechanisms associated with oxaliplatin resistance include intracellular transport and detoxification, alterations in DNA repair mechanisms and cell death mechanisms. Failure in the oxaliplatin induced DNA damage repair usually caused cell death activation. Therefore, the activation of cell death pathways influences the efficacy of oxaliplatin. Oxaliplatin exert its cytotoxic effect mainly by activating the intrinsic apoptosis pathway (33). The intrinsic apoptosis pathway is regulated by the BCL-2 family, which contains pro-apoptotic (BAD, BAX, BAK) and anti-apoptotic members (BCL-2, BCL-xl, MCL-1). It has been reported that loss of pro-apoptotic BAX decreases while down-regulation of the anti-apoptotic BCL-2 and BCL-xl increases sensitivity to oxaliplatin (34,35). Consistently, we found that the anti-apoptotic BCL-2 was up-regulated and the pro-apoptotic BAD and BAX was down-regulated in Lv-shSEMA3F cells compared with Lv-control cells. However, there was no difference in the expression of P53. Moreover, we found that knock-down of SEMA3F increased promotes invasion and EMT of CRC cells. It should be noticed that the increased invasion of CRC cells might be a combined effect of the elevated invasion capacity and cell viability. During the process of EMT, cells undergo loss of epithelial cell-cell junctions, re-organization of cytoskeleton and molecular changes such as decrease the expression of epithelial markers and increase the expression of mesenchymal markers. Besides invasion, metastasis and stemness, EMT also plays important role in drug resistance in many cancers, such as breast cancer, lung cancer, pancreatic cancer and CRC (18,36). Many signals in the tumor microenvironment, such as oxygen tension, cytokines and growth factors can induce EMT in cancer (37). We propose that SEMA3F increases chemosensitivity to oxaliplatin of CRC cells at least partially through activating apoptosis pathways and inhibiting the EMT process.
In conclusion, the present study demonstrated that SEMA3F induces apoptosis, inhibits EMT and increases chemosensitivity to oxaliplatin of CRC cells. These results provides evidence for the development of novel therapeutic intervention of CRC based on the axon guidance repulsive SEMA3F.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation for Young Scientist of China (No. 81602097, 81402460) and Key research of the 309th hospital (No.2016ZD-009). We thank for the unselfish help from Pro. Xiu-Wu Bian (Institute of Pathology and Southwest Cancer Center, Southwest Hospital, Third Military Medical University, China).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was carried out in according to the principles of the Helsinki Declaration and approved by the Ethical Committee of the Third Military Medical University (No. 2013062). Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rehman M, Tamagnone L. Semaphorins in cancer: biological mechanisms and therapeutic approaches. Semin Cell Dev Biol 2013;24:179-89. [Crossref] [PubMed]
- Tamagnone L. Emerging role of semaphorins as major regulatory signals and potential therapeutic targets in cancer. Cancer Cell 2012;22:145-52. [Crossref] [PubMed]
- Serini G, Bussolino F, Maione F, et al. Class 3 semaphorins: physiological vascular normalizing agents for anti-cancer therapy. J Intern Med 2013;273:138-55. [Crossref] [PubMed]
- Sekido Y, Bader S, Latif F, et al. Human semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc Natl Acad Sci U S A 1996;93:4120-5. [Crossref] [PubMed]
- Xiang RH, Hensel CH, Garcia DK, et al. Isolation of the human semaphorin III/F gene (SEMA3F) at chromosome 3p21, a region deleted in lung cancer. Genomics 1996;32:39-48. [Crossref] [PubMed]
- Lantuéjoul S, Constantin B, Drabkin H, et al. Expression of VEGF, semaphorin SEMA3F, and their common receptors neuropilins NP1 and NP2 in preinvasive bronchial lesions, lung tumours, and cell lines. J Pathol 2003;200:336-47. [Crossref] [PubMed]
- Staton CA, Shaw LA, Valluru M, et al. Expression of class 3 semaphorins and their receptors in human breast neoplasia. Histopathology 2011;59:274-82. [Crossref] [PubMed]
- Drenberg CD, Livingston S, Chen R, et al. Expression of Semaphorin 3F and Its Receptors in Epithelial Ovarian Cancer, Fallopian Tubes, and Secondary Müllerian Tissues. Obstet Gynecol Int 2009;2009:730739.
- Zhou ZH, Rao J, Yang J, et al. SEMA3F prevents metastasis of colorectal cancer by PI3K-AKT-dependent down-regulation of the ASCL2-CXCR4 axis. J Pathol 2015;236:467-78. [Crossref] [PubMed]
- Bielenberg DR, Hida Y, Shimizu A, et al. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest 2004;114:1260-71. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Moghimi-Dehkordi B, Safaee A. An overview of colorectal cancer survival rates and prognosis in Asia. World J Gastrointest Oncol 2012;4:71-5. [Crossref] [PubMed]
- Jin K, Gao W, Lu Y, et al. Mechanisms regulating colorectal cancer cell metastasis into liver Oncol Lett 2012;3:11-15. (Review). [PubMed]
- Bavykin AS, Korotaeva AA, Poyarkov SV, et al. Double siRNA-targeting of cIAP2 and LIVIN results in synergetic sensitization of HCT-116 cells to oxaliplatin treatment. Onco Targets Ther 2013;6:1333-40. [Crossref] [PubMed]
- Martinez-Balibrea E, Martínez-Cardús A, Ginés A, et al. Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol Cancer Ther 2015;14:1767-76. [Crossref] [PubMed]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009;9:265-73. [Crossref] [PubMed]
- Fischer KR, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015;527:472-6. [Crossref] [PubMed]
- Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015;527:525-30. [Crossref] [PubMed]
- Wu F, Zhou Q, Yang J, et al. Endogenous axon guiding chemorepulsant semaphorin-3F inhibits the growth and metastasis of colorectal carcinoma. Clin Cancer Res 2011;17:2702-11. [Crossref] [PubMed]
- Rao J, Zhou ZH, Yang J, et al. Semaphorin-3F suppresses the stemness of colorectal cancer cells by inactivating Rac1. Cancer Lett 2015;358:76-84. [Crossref] [PubMed]
- Lerman MI, Minna JD. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res 2000;60:6116-33. [PubMed]
- Ping YF, Yao XH, Jiang JY, et al. The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signalling. J Pathol 2011;224:344-54. [Crossref] [PubMed]
- Kusy S, Nasarre P, Chan D, et al. Selective suppression of in vivo tumorigenicity by semaphorin SEMA3F in lung cancer cells. Neoplasia 2005;7:457-65. [Crossref] [PubMed]
- Potiron VA, Sharma G, Nasarre P, et al. Semaphorin SEMA3F affects multiple signaling pathways in lung cancer cells. Cancer Res 2007;67:8708-15. [Crossref] [PubMed]
- Nasarre P, Kusy S, Constantin B, et al. Semaphorin SEMA3F has a repulsing activity on breast cancer cells and inhibits E-cadherin-mediated cell adhesion. Neoplasia 2005;7:180-9. [Crossref] [PubMed]
- Joseph D, Ho SM, Syed V. Hormonal regulation and distinct functions of semaphorin-3B and semaphorin-3F in ovarian cancer. Mol Cancer Ther 2010;9:499-509. [Crossref] [PubMed]
- Bollard J, Massoma P, Vercherat C, et al. The axon guidance molecule semaphorin 3F is a negative regulator of tumor progression and proliferation in ileal neuroendocrine tumors. Oncotarget 2015;6:36731-45. [PubMed]
- Suzuki T, Nishio K, Tanabe S. The MRP family and anticancer drug metabolism. Curr Drug Metab 2001;2:367-77. [Crossref] [PubMed]
- Singh S. Cytoprotective and regulatory functions of glutathione S-transferases in cancer cell proliferation and cell death. Cancer Chemother Pharmacol 2015;75:1-15. [Crossref] [PubMed]
- Nakayama H, Bruneau S, Kochupurakkal N, et al. Regulation of mTOR Signaling by Semaphorin 3F-Neuropilin 2 Interactions In Vitro and In Vivo. Sci Rep 2015;5:11789. [Crossref] [PubMed]
- Bhattacharya R, Ye XC, Wang R, et al. Intracrine VEGF Signaling Mediates the Activity of Prosurvival Pathways in Human Colorectal Cancer Cells. Cancer Res 2016;76:3014-24. [Crossref] [PubMed]
- Gu Y, Wang Q, Guo K, et al. TUSC3 promotes colorectal cancer progression and epithelial-mesenchymal transition (EMT) through WNT/β-catenin and MAPK signalling. J Pathol 2016;239:60-71. [Crossref] [PubMed]
- Tao Z, Goodisman J, Penefsky HS, et al. Caspase activation by anticancer drugs: the caspase storm. Mol Pharm 2007;4:583-95. [Crossref] [PubMed]
- Gourdier I, Del Rio M, Crabbé L, et al. Drug specific resistance to oxaliplatin is associated with apoptosis defect in a cellular model of colon carcinoma. FEBS Lett 2002;529:232-6. [Crossref] [PubMed]
- Hayward RL, Macpherson JS, Cummings J, et al. Enhanced oxaliplatin-induced apoptosis following antisense Bcl-xl down-regulation is p53 and Bax dependent: Genetic evidence for specificity of the antisense effect. Mol Cancer Ther 2004;3:169-78. [PubMed]
- Xu T, Zhang J, Chen W, et al. ARK5 promotes doxorubicin resistance in hepatocellular carcinoma via epithelial-mesenchymal transition. Cancer Lett 2016;377:140-8. [Crossref] [PubMed]
- Wu G, Wilson G, George J, et al. Overcoming treatment resistance in cancer: Current understanding and tactics. Cancer Lett 2017;387:69-76. [Crossref] [PubMed]


