
Editorial to “Palbociclib and letrozole in advanced breast cancer”
Palbociclib is a first-in-class inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6) that inhibits cell proliferation and DNA synthesis by preventing cell-cycle progression from G1 to S phase (1). In cell line models of ER-positive breast cancer, estrogen activates CDK4/6 via cyclin D1, resulting in hyperphosphorylation of retinoblastoma (Rb) gene product, which leads to entry of the cell cycle (2). Endocrine resistance has been shown to be depends on cyclin D1 and CDK4/6 in vitro. Based on the impressive progression-free survival (PFS) found in the phase 2 study PALOMA-1 (3), palbociclib was granted accelerated approval in 2015 by the Food and Drug Administration (FDA) for treatment of estrogen-receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer (ABC).
Key results of PALOMA-2
PALOMA-2, is a double-blind, placebo-controlled, randomized phase 3 study of palbociclib plus aromatase inhibitor (AI) letrozole in women with ER-positive, HER2-negative ABC who had no prior treatment for advanced disease (4). Patients were randomized in a 2:1 ratio to received palbociclib plus letrozole or placebo plus letrozole. The primary end point was PFS. Secondary end points included overall survival (OS), objective response (OR), clinical benefit response (CBR) and safety.
The study successfully randomized 666 women within 17 months. Median duration of follow up was 23 months. The primary endpoint was met—the addition of palbociclib to letrozole, as compared with placebo-letrozole, increased the median PFS from 14.5 months [95% confidence interval (CI), 12.9 to 17.1] to 24.8 months (95% CI, 22.1 to not estimable) [hazard ratio (HR) 0.58, 95% CI, 0.46 to 0.72; P<0.001]. The treatment efficacy was confirmed by a blinded independent review committee. Subgroup analyses of PFS according to study stratification factors and other baseline characteristics confirmed a consistent benefit across all subgroups evaluated (HR ranges, 0.35–0.67). In short, the benefit was seen irrespective of race, disease-free survival, visceral involvement, prior hormonal therapy, the type of recent hormonal therapy, or prior chemotherapy.
The rate of OR for all randomly assigned patients in palbociclib-letrozole group versus placebo-letrozole group was 42.1% (95% CI, 37.5 to 46.9) and 34.7% (95% CI, 28.4 to 41.3) (odds ratio 1.4, 95% CI, 0.98 to 2.01; P=0.06), and among patients with measurable disease according to RECIST the corresponding rate of OR was 55.3% (95% CI, 49.9 to 60.7) and 44.4% (95% CI, 36.9 to 52.2) respectively (odds ratio 1.55, 95% CI, 1.05 to 2.28; P=0.03). The rate of CBR among all patients randomized was 84.9% (95% CI, 81.2 to 88.1) for palbociclib-letrozole group and 70.3% (95% CI, 63.8 to 76.2) for placebo-letrozole group (odds ratio 2.39, 95% CI, 1.58 to 3.59; P<0.0010). Similarly, in those with measurable disease, the rate of CBR in these two groups was 84.3% (95% CI, 80.0 to 88.0) and 70.8% (95% CI, 63.3 to 77.5) respectively (odds ratio 2.23, 95% CI, 1.39 to 3.56; P<0.001).
The more common grade 3 or 4 adverse events (AEs) in the palbociclib-letrozole group were neutropenia (66%), leukopenia (25%), and anemia (5%). Febrile neutropenia occurred in 1.8% of patients in this group. Serious (grade 4) AEs of any cause occurred in 19.6% of patients in the palbociclib-letrozole group and 12.6% of patients in the placebo-letrozole group. Permanent treatment discontinuation due to AEs was 9.7% in the palbociclib-letrozole group and 5.9% in the placebo-letrozole group.
Discussion
PALOMA-2 showed superior efficacy for palbociclib plus letrozole in first line treatment of ER-positive HER2-negative ABC with an unprecedented PFS of 2 years. Subgroup analysis suggested all patients, including those with prior exposure to hormonal therapy or chemotherapy, derived benefit from this approach. This study confirms the important roles of CDK4/6 inhibition in the management of ER-positive HER2-negative ABC. CDK 4/6 inhibition in combination with hormonal therapy is clearly a new standard.
The results of PALOMA-2 echo those of PALOMA-1 that led to FDA approval. PALOMA-1 differs from PALOMA-2, besides being a phase 2 trial, in that it adopted a 1:1 randomization. There were also small differences in the subgroup analysis, such as inclusion of the newly diagnosed metastatic disease subgroup. Nevertheless, both studies showed significant survival benefit of palbociclib and similar toxicity profiles. Longer follow up is needed for the effect on overall survival.
Ribociclib is another CDK 4/6 inhibitor in clinical development. MONOLEESA-2 is a double-blind, placebo-controlled, phase 3 study of ribociclib plus letrozole and mirrors PALOMA-2 for the target patient population (5). Patients were randomized 1:1 to ribociclib-letrozole or placebo-letrozole. The study, similar to PALOMA-2, demonstrated that the addition of ribociclib to letrozole significantly improved PFS giving a comparable HR of 0.56 for disease progression or death (P<0.001), as well as a similar overall response rate. Of note, PALOMA-2 included more Asians than MONALEESA-2, and recruited 20% more patients who had endocrine resistance (DFS ≤12 months) at baseline. Although neutropenia and leukopenia were the most common AEs for both CDK 4/6 inhibitors and the rate of occurrence was similar in both trials, there were some differences in the toxicity profile worth noticing. It appeared that anemia, thrombocytopenia and possibly stomatitis were more common in patients receiving palbociclib-letrozole and most of these AEs were mild. On the other hand, elevated alanine aminotransferase and aspartate aminotransferase have been observed in patients given ribociclib-letrozole and grade 3 or 4 transaminitis could happen in up to 9% of these patients. Prolonged QTcF was also a concern for a small proportion of patients in MONALEESA-2. Although palbociclib and ribociclib have comparable spectrum of CDK activity (6), the minor disparities in their chemical structure might explain these differences in toxicity profile. The median follow up time for MONALEESA-2 was only 15.3 months and more mature PFS data is awaited. The comparison between these two clinical trials is summarized in Table 1.
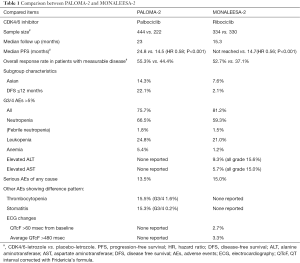
Full table
The major toxicity of palbociclib was neutropenia and it led to dose reduction in 29% of patients. Its significance wasn’t discussed in this paper. PALOMA-3 is a study of palbociclib in combination with fulvestrant in patients who progressed on first line hormonal therapy (7). Detailed analysis showed that dose modification of palbociclib for grade 3 and 4 neutropenia had no adverse effect on PFS (8). PALAMO3 also demonstrated that the quality of life (QoL) among patients on palbociclib was better maintained than those given placebo. Together with a low frequency of febrile neutropenia, all these results supported palbociclib is a drug well tolerated.
AI has been the standard of care for first line treatment of patients with ER-positive HER2-negative ABC. Besides CDK 4/6 inhibitors, recently this standard is also challenged by another hormonal therapy fulvestrant. Fulvestrant is a selective ER degrader. In the phase 3 FALCON trial, patients given monthly injection of fulvestrant at 500 mg had significantly longer median PFS of 16.6 months (95% CI, 13.83 to 20.99) compared with those of 13.8 months (95% CI, 11.99 to 16.59) given oral anastrozole 1 mg daily (HR 0.797, 95% CI, 0.637 to 0.999; P=0.0486) (9). This approach showed that the ceiling PFS ceiling of hormonal therapy could be stretched to 20 months in some patients.
With the emergence of new treatment options and increasing financial burden associated with them, the practical questions would be how to choose the first line hormonal therapy. Current guidelines lay the general clinical principle that treatment recommendation should be based on if the patient is naïve to endocrine therapy, the type of adjuvant therapy, length of disease free interval and if disease relapsing less than 12 months from the end of adjuvant AI (10,11). More updated and detailed guidelines are anticipated later this year in light of recent breakthrough findings.
At the age of personalized medicine, we would like study of predictive biomarkers could enlighten us on how to optimize treatment choice. Biomarkers studied for palbociclib include cyclin D1 amplification and p16 loss in PALOMA-1 (3), ER expression, Rb level, p16 loss, cyclin D1 amplification and Ki67 index in PALOMA-2 (12), as well as hormone-receptor expression level, PIK3CA mutation status, and plasma circulating tumor DNA ESR1 mutation status in PALOMA-3 (13,14). Unfortunately, all these analyses were negative. A better understanding in biology of endocrine resistance and study of fresh biopsy at the time of disease recurrence or progression might provide valuable insight in this field.
A proportion of patients given adjuvant hormonal therapy relapse eventually. The impressive response in metastatic setting and decent QoL data of palbociclib opens door for the development of this drug in adjuvant setting. A number of adjuvant trials for palbociclib are ongoing. PALLAS evaluates the outcome of adding 2 years of palbociclib to standard endocrine therapy (NCT02513394). PENELOPE-B studies the role of adding palbociclib to standard endocrine therapy in patients with high relapse risk after neoadjuvant chemotherapy (NCT01864746). These studies should also provide insights on tolerability of this drug in the healthy population.
Palbociclib is a first-in-class CDK 4/6 inhibitor in clinical use. It is a game changer for first line ER-positive HER2-negative ABC and for patients with endocrine resistance. There is an unmet need for biomarker of response to guide management decision. Further studies on the benefit of continuing CDK 4/6 inhibitor beyond progression or optimal treatment strategy after resistance to CDK 4/6 inhibitor would also be needed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Associate Editor-in-Chief Binghe Xu (Department of Medical Oncology at the Cancer Hospital and Institute, Chinese Academy of Medical Sciences (CAMS), and Peking Union Medical College (PUMC) in Beijing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.21). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roberts PJ, Bisi JE, Strum JC, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst 2012;104:476-87. [Crossref] [PubMed]
- Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009;11:R77. [Crossref] [PubMed]
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25-35. [Crossref] [PubMed]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-36. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med 2016;375:1738-48. [Crossref] [PubMed]
- Chen P, Lee NV, Hu W, et al. Spectrum and Degree of CDK Drug Interactions Predicts Clinical Performance. Mol Cancer Ther 2016;15:2273-81. [Crossref] [PubMed]
- Turner NC, Ro J, André F, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med 2015;373:209-19. [Crossref] [PubMed]
- Verma S, Bartlett CH, Schnell P, et al. Palbociclib in Combination With Fulvestrant in Women With Hormone Receptor-Positive/HER2-Negative Advanced Metastatic Breast Cancer: Detailed Safety Analysis From a Multicenter, Randomized, Placebo-Controlled, Phase III Study (PALOMA-3). Oncologist 2016;21:1165-75. [Crossref] [PubMed]
- Robertson JF, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016;388:2997-3005. [Crossref] [PubMed]
- Rugo HS, Rumble RB, Macrae E, et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 2016;34:3069-103. [Crossref] [PubMed]
- Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO international consensus guidelines for Advanced Breast Cancer (ABC 3). Breast 2017;31:244-59. [Crossref] [PubMed]
- Finn R, Jiang Y, Rugo Y, et al. Biomarker analyses from the phase 3 PALOMA-2 trial of palbociclib (P) with letrozole (L) compared with placebo (PLB) plus L in postmenopausal women with ER + /HER2– advanced breast cancer (ABC). Ann Oncol 2016;27:LBA15. [Crossref]
- Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425-39. [Crossref] [PubMed]
- Turner NC, Jiang Y, O'Leary B, et al. Efficacy of palbociclib plus fulvestrant (P+F) in patients (pts) with metastatic breast cancer (MBC) and ESR1 mutations (mus) in circulating tumor DNA (ctDNA). J Clin Oncol 2016;34:abstr 512.


