Third generation gold nanoplatform optimized for radiation therapy
Background
Among the myriad of nanoparticles being used for medical applications, metallic gold nanoparticles stand out because of their excellent biocompatibility, ease of surface modification, size tunability and attractive physicochemical properties (1-4). Gold nanoparticles (GNPs) of different sizes have shown a tremendous potential in photothermal ablation, molecular diagnostics, drug delivery and radiation therapy applications (5-12). The tunable optical properties and functionalities of gold nanoparticles for biomedical application has opened new ways to detect and treat cancer (3). Owing to its high atomic number (Z), gold nanoparticles are particularly attractive as radiosensitizers in radiation therapy (13-15). The interaction of X-rays with high Z-materials is well documented and it has been established that radiation dose enhancement is one possible result. It has been hypothesized that low energy electrons, emitted after photoelectric absorption of X-rays, can deliver a lethal energy dose within a short range in biological media leading to increased cell kill thus enhancing the efficacy of radiation therapy (16-18).
A few applications of gold nanoparticles in radiation therapy have been reported (9,14,19). The pioneering work by Hainfield used 1.9 nm bare gold nanoparticles and 15 nm PEGylated gold nanoparticles for radiation dose enhancement (10,14). PEGylated gold nanoparticles of 30 nm have also been used for radiation therapy (20). However, in these studies nanoparticle design has not been optimized for clinical translation. The size and charge of the nanoparticles play a critical role in biodistribution, targeted binding and clearance of the nanoparticles from the animal body (21). Small size gold nanoparticles (<6 nm) will tend to be cleared via renal excretion within a few minutes post intravenous administration (22-24). Larger nanoparticles are known to be accumulated in the RES (Reticuloendothelial System) leading to less tumor accumulation and undesirable side effects upon irradiation with X-rays (i.e., enhanced radiation damage to the non-targeted RES organs) (25-28). Importantly, the lack of in vivo monitoring of nanoparticles is a major drawback for these reported formulations. Micro-CT (computed tomography) provides one modality for imaging of gold nanoparticles, however the typical concentrations required for adequate contrast in the tumor is not currently feasible.
A better understanding of the interaction of nanomaterials in biological systems has laid a foundation for designing smart nanoparticles for various biomedical applications. Engineered nanostructures that are capable of long circulation in the body, delivering therapeutics to targeted sites requires an understanding of the physico-chemical interactions of a synthetic nanoparticle with biological systems. A careful nanoparticle design should consider the disease type, microenvironment of the diseases site, mode of interaction with non-target organs and the fate of these nanoparticles in in vivo environment. Three ‘S’ of nanoparticles parameters: size, shape and surface charge are the fundamental parameters which decide the success of any nanoparticle based platform in living systems (21,29). Thus it is imperative to assess these three properties before engineering a nanoparticle for a particular biological application.
The first generation of nanomaterials included investigations on the surface modifications to assess in vitro uptake and cytotoxicity without taking biological challenges into consideration (30,31). The second generation of nanoparticle designs was primarily focused on the stealth behavior and active targeting (32-34). Currently, third generation nanoparticles are being engineered based on the difficult lessons learned from the previous two generation taking into account all the limitations associated with them for biological applications (35).
The focus of this manuscript is in developing a 3rd generation gold nanoparticles based platform for application in radiation oncology. Our group has already established the use of gold nanoparticles in radiation dose enhancement in vitro with HeLa cell line (36,37). Using these rational design considerations, we have developed a 3rd generation gold nanoparticle formulation (called as AuRadTM) for application in radiation dose enhancement with concomitant possibility of optical imaging in small animals for nanoparticles tracking and simultaneous disease progression monitoring. The conjugation of optical probe is a proof of concept for successful surface conjugation to various imaging modalities like Magnetic Resonance (MR), Positron Emission Tomography (PET) or CT imaging. The salient features of this versatile platform includes (I) an ultrasmall size of core GNPs; (II) PEGylation of core GNPs with heterobifuntional PEG (pGNPs); (III) a variety of functional groups on the PEG tip; (IV) a covalently conjugated fluorophore on the PEG tip linked to GNPs and (V) high radiation dose enhancement. As synthesized ultrasmall 2-3 nm gold nanoparticles were PEGylated with a mixture of heterobifunctional PEG for surface functionality. The hydrodynamic diameter of the GNPs was maintained around 11-12 nm to extend circulation by bypassing rapid renal excretion while avoiding excessive RES accumulation. The functional groups on the surface of the pGNPs were used for conjugating an optical probe (Aleaxafluor 647) for fluorescence optical imaging. These ultrasmall pGNPs, designed for maximal tumor uptake and optical imaging has great potential in radiation therapy and a successful translation to clinics in near future
Materials and methods
Materials
Gold (III) Chloride trihydrate (HAuCl4.3H2O), Tetrakis (hydroxymethyl) phosphnium chloride (80% solution in water), Sodium hydroxide and HPLC grade water were purchased from Sigma Aldrich. Solvents like dimethyl sulfoxide (DMSO) were procured from Sigma and used without further purifications. Mono-functional mPEG-thiol (Mw: 2,000 Da) and hetero bi-functional anime-PEG-thiol (Mw: 3,400 Da) and carboxymethyl-PEG-thiol (Mw: 2,000 Da) were purchased from Layson Bio Inc. Alexa Fluor 647 (AF647) carboxylic acid, succinimidyl ester was purchased from Invitrogen (Carlsbad, CA). Microfuge membrane-filters (NANOSEP 100K OMEGA) are a product of Pall Corporation. Cellulose dialysis membrane cellulose membrane with a cutoff size of 12-14 kDa was purchased from Sigma Aldrich. Cell culture reagents like media DMEM, Penstrep (antibiotic) and fetal bovine serum were purchased from Invitrogen. The cancer cell line HeLa was obtained from ATCC, VA and cultured according to instructions supplied by the vendor. For cell viability assay CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) reagent was procured from Promega.
Synthesis of gold nanoparticles and PEGylation
The synthesis of PEGylated gold nanoparticles was carried out in a two-step process. Gold colloids synthesis typically involves the reduction of gold salts in the presence of surfactants or stabilizers. In this study, colloidal gold nanoparticles were prepared by reduction of chloroauric acid, using tetrakis (hydroxymethyl) phosphonium chloride (THPC), as the reducing agent. The THPC capped gold nanoparticles were prepared by a well-established method reported earlier by Pham et al. with slight modifications (38). Briefly, to 45 mL of HPLC grade water, 0.5 mL of freshly prepared 1 M NaOH and 1 mL of THPC solution (prepared by adding 12 µL of 80% THPC in water to 1 mL of HPLC water) were added and stirred vigorously for 5 min at room temperature. 2 mL of 25 mM HAuCl4 was added rapidly to the stirring mixture. While mixing, the color of the mixture changed from yellow to dark brown, indicating the formation of THPC capped gold nanoparticles. The reaction mixture was further stirred for 15 min. The reduction of auric chloride in the presence of THPC results in the formation of ultrasmall gold nanoparticles with diameters ~2 nm each with a net negative surface charge.
The second step is the PEGylation of the as synthesized gold nanoparticles by a simple ligand exchange process using a mixture of functional derivatives of thiolated PEG. Briefly, 45 mL of as prepared THPC capped GNPs were incubated with 2 mL of 3.75 mg/mL m-PEG-thiol, 2 mL of carboxymethyl-PEG-thiol and 4 mL of amine-PEG-thiol. The reaction mixture was gently stirred overnight at room temperature. To remove unreacted PEG and reactants, the mixture was then dialyzed against distilled water for 24 hrs using a cellulose membrane with a cut-off size of 12-14 kDa. Following dialysis the nanoparticles solution was sterile filtered using a 0.45 µm syringe filter and lyophilized to obtain dried pGNPs and stored at 4 °C for future use.
AF647conjugation to PEGylated gold nanoparticles
The succinimidyl ester of AF647 was covalently conjugated to the amine groups of the PEG on the surface of the pGNPs. 2 mg of pGNPs were dispersed in 1 mL of carbonate buffer (0.1 M, pH 8.8) followed by addition of 5 µL of 10 mg/mL AF647 succinimidyl ester in DMSO. The reaction mixture was gently stirred for 2 hrs at room temperature. The solution was centrifuged at 7,000 rpm for 45 min at 4 °C using 100 kDa centrifugal spin filters to remove any unbound AF647 from the solution. The precipitated AF647 conjugated pGNPs (AF647-pGNPs) were washed three times with HPLC grade water to ensure complete removal of free fluorophore. The nanoparticles were further lyophilized to powdered form to store for future use.
UV-Visible absorbance
The absorption spectra were collected using an Agilent model 8453 UV-Vis scanning spectrophotometer over a wavelength range from 300 to 800 nm. The samples were measured against water as reference. All samples were used as prepared and loaded into a quartz cell for measurements.
Photoluminescence (PL) spectroscopy
The emission spectra were collected using a Fluoromax 4 Spectrofluorometer (Jobin Yvon). All the samples were dispersed in water and loaded into a quartz cell for measurements.
Transmission electron microscopy (TEM)
Transmission electron microscopy (TEM) images were obtained using a JEOL model JEM-1000 microscope at an acceleration voltage of 80 kV. The specimens were prepared by drop casting the sample dispersion onto an amorphous carbon coated 300 mesh copper grid, which was placed on a filter paper to absorb the excess solvent.
Dynamic light scattering (DLS) measurements were performed by using 90 Plus zeta sizer (Brookhaven Inc, NY) for measuring the hydrodynamic diameter of the pGNPs nanoparticles in a diluted sample placed in a 3 mL cuvette. Zeta potential measurements were also done using the same instrument.
Cell staining studies
For in vitro imaging with AF647-pGNPs, the human cervical cancer cell line HeLa was cultured in Dulbecco minimum essential media (DMEM) with 10% fetal bovine serum (FBS), 1% penicillin, and 1% amphotericin B. The day before nanoparticles treatment, cells were seeded in 35 mm culture dishes. On the treatment day, the cells, at a confluence of 70-80% in serum-supplemented media, were treated with the nanoparticles at a specific concentration (100 µL/1 mL media) for two hours at 37 °C.
Cell viability assay
The HeLa cells were dispensed into a 96-well flat-bottom microtiter plate (~10,000 cells/well) and allowed to attach overnight using the DMEM medium with 10% FBS. The MTS assay was carried out as per manufacturer’s instructions (PROMEGA). It is based on the absorbance of formazan (produced by the cleavage of MTS by dehydrogenases in living cells), the amount of which is directly proportional to the number of live cells. In brief, after 24 h treatment with the pGNPs, media was changed and 150 µL of MTS reagent was added to each well and mixed. The absorbance of the mixtures at 490 nm was measured. The cell viability was calculated as the ratio of the absorbance of the sample well to that of the control well and expressed as a percentage. Tests were performed in quadruplicate. Each point represents the mean ± SD (bars) of replicates from one representative experiment. (See supporting information).
Confocal microscopy
Confocal microscopy images were obtained using a Zeiss LSM700 confocal microscope (Carl Zeiss, Europe) with laser excitation at 488 nm using a 60× oil immersion objective. All images were taken under exact same conditions of laser power (50.99%), aperture (1.4), pin hole (2 airy), offset (–90.70), scanning speed (400 Hz), and scanning area (238 µm × 238 µm).
In vitro X-ray irradiation
For irradiation experiments, 6 well cell culture plate was seeded with 40,000 cells/well in DMEM media supplemented with 10% FBS one day prior to treatment with nanoparticles. Two sets of 6 well plates were prepared for each sample in which one plate was irradiated whereas the other non-irradiated plate served as negative control. Cells were treated with two different concentrations of pGNPs (0.5 and 2 mg/mL), whereas one set of well with no nanoparticles kept as controls. Nanoparticles were incubated for 24 hrs following which the cells were irradiated. Irradiation experiments were carried out using Seifert ISOVOLT 225M2 X-ray source (Seifert X-ray, Lewistown, PA) on a SARRP platform operating at 220 kvp and 13 mA using a 0.15 mm copper filter (39). The dose rate at a source-subject distance (SSD) of 35 cm was 5.45 Gy/min (2 Gy in 22 s).
Clonogenic survival assay
Clonogenic cell survival assays were performed for evaluating the therapeutic effect of radiation on the cell survival with and without pGNPs. After irradiation, cells were incubated for another 24 hrs. After this period, the cells were trypsinized and seeded in triplicates in 100 mm petriplates for 14 days. After 14 days of incubation, the cells were washed with PBS and stained with trypan blue in methanol. All colonies with over 50 cells were counted. The relative cell surviving fraction was calculated by, using equation 1 and 2, dividing the number of colonies of irradiated cells by the cells plated, with a correction for the plating efficiency (PE) (40).
PE is the ratio of the number of colonies to the number of cells seeded whereas the number of colonies that arise after treatment of cells, expressed in terms of PE, is called the surviving fraction (SF).
Results
Figure 1 shows the schematic illustration of the nanoparticles design and synthesis. The schematic represents the PEGylation of the as synthesized gold nanoparticles with heterobifunctional PEG and further conjugation with AF647. The amine groups present on the tip of the functionalized PEG on nanoparticles surface were utilized for covalently conjugating the AF647 to the gold nanoparticles.
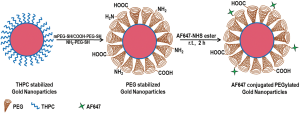
Figure 2 (left panel) shows a representative TEM image of the as synthesized THPC capped gold nanoparticles. As can be visualized from the TEM image, the nanoparticles have a spherical morphology with a narrow size distribution. Figure 2 (right panel) shows a TEM image of THPC gold nanoparticles after PEGylation. The PEGylation process maintained the integrity of the core gold nanoparticles as the size of individual gold nanoparticles remained same. The polymeric PEG molecules which forms a dense layer around the metallic gold nanoparticles cannot be visualized in the TEM without additional negative staining. However, we have confirmed this with the DLS studies. DLS has shown the nanoparticles to be of unimodal distribution with the maximum percentage intensity to be of 12 nm in size. Since DLS represents the hydrodynamic diameter, it becomes quite evident that after ligand exchange with PEG, the overall diameter of the nanoparticles becomes 12 nm out of which approx. 6-7 nm is contributed by the PEG molecule on the surface of GNPs. PEG with a molecular weight of 2,000 Da adds an additional 6-8 nm in its stretched linear form (26). This increase in size with the addition of PEG is consistent with the DLS data.

The long-term stability of the pGNPs was established over six months with DLS and surface plasmon resonance. Figure 3A shows the comparative DLS data of the THPC capped GNPs and pGNPs measured over 6 months time. The DLS data clearly indicates a linear increase in the size of the THPC capped GNPs over the incubation time period which can be attributed to Ostwald ripening and further aggregation of nanoparticles. However, PEGylated nanoparticles showed an insignificant change in the size of the nanoparticles over the same time period indicating a higher degree of stabilization of the gold nanoparticles by the conjugated PEG on the nanoparticles surface. The observation of long-term stability was further confirmed with the characteristic surface plasmon behavior of the gold nanoparticles which is very well known to be dependent on the size of the gold nanoparticles. Figure 3 (right panel) shows the extinction spectra of the gold THPC capped GNPs and pGNPs. Freshly prepared THPC capped GNPs shows a surface plasmon resonance (SPR) band around 515-520 nm which is the characteristic plasmonic property of small size colloidal gold nanoparticles. The same sample, when stored, as is, for six months without any additional stabilization showed a bathochromic shift of the SPR peak along with a second prominent peak centered at 680-700 nm. This second peak for the 6-month old THPC capped GNPs corresponds to aggregated clusters of GNPs (41).
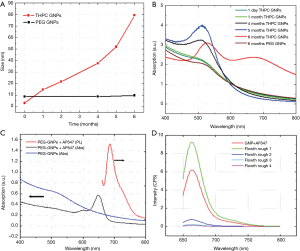
Similarly 4- and 5-month old samples showed a bathochromic shift from the freshly prepared sample with an increased intensity, indicating the increase in the size of the nanoparticles by Ostwald ripening and aggregation. However, pGNPs exhibited the same plasmon behavior after six months of storage under similar conditions. Gold nanoparticles are known to exhibit characteristic SPR phenomena which is highly dependent on the size of nanoparticles. This SPR behavior of THPC GNPs and pGNPs was correlated with the DLS data we obtained for these nanoparticles.
Optical imaging imparts an added modality for tracking the nanoparticles in vitro and in vivo. For this purpose, the conjugation of AF647 to the pGNPs surface via the functional groups present on the tip of the PEG molecule provides optical signature which can be tracked using optical spectroscopy. The photophysical characterization of the AF647-pGNPs is shown in Figure 3. Figure 3C shows the absorption and fluorescence spectra of the conjugated fluorophore. Absorption spectra of the pGNPs showed a typical SPR peak as explained in previous section whereas once the AF 647 was conjugated to these pGNPs, there is a prominent absorption peak with λmax650 nm which is characteristic optical signature of AF647. With an excitation wavelength of 633 nm, AF647-pGNPs showed an emission peak at λmax668 nm. To ensure that all these optical signatures are only from the AF 647 molecules conjugated to pGNPs and not from free AF647, we have used centrifugal spin filtration method to separate the unbound AF647 from the reaction mixture. The flow-through obtained after separating the AF647-pGNPS was measured for free AF647 by fluorescence studies. Figure 3D shows fluorescence emission peaks for each iterative flow-through at λmax663 nm indicating the presence of unbound AF647 in solution. The repeated washing with water removed any unbound AF647 from the nanoparticles and this was confirmed by the fluorescence spectra of the flow-through after fourth washing. In addition, the high fluorescence intensity obtained with the final washed AF647-pGNPs demonstrated high degree of AF 647 conjugation.
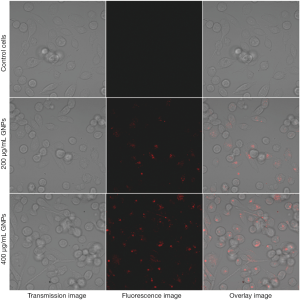
To demonstrate the imaging capability of the AF647-pGNPs for optical tracking of the nanoparticles in in vitro and in vivo systems, the AF647-pGNPs are used to label the cervical cancer HeLa cell line. Figure 4 shows confocal fluorescence images of live cells incubated with two different concentrations of AF647-GNPs for 2 hrs. Both concentrations of the nanoparticles have a robust uptake as can be visualized by the strong cellular staining outside the nucleus in the ER region. No morphological damage was observed for HeLa cells, indicating low cytotoxicity of these nanoparticles.
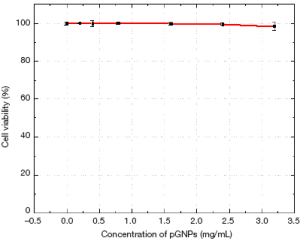
To estimate if these functional nanoparticles are biocompatible and do not cause any physiological damage to the cells, the AF647-pGNPs were evaluated by MTS assay. The nanoparticles were treated with cells at different concentrations for a period of 24 hrs with concentrations as high as 2.5 mg/mL of nanoparticles. Figure S1 (see supplementary information) shows the results of the MTS assay with HeLa cells treated with AF647-pGNPs nanoparticles. One can observe that cells retained more than 90% viability even in the presence of a highest nanoparticle loading of 2.5 mg/mL for 24 h. The low in vitro cytotoxicity of AF647-pGNPs may be attributed to several factors, including the non-reactive nature of the metallic gold nanoparticles as well as the presence of the highly hydrophilic, non-immunogenic and biocompatible PEG layer on the nanoparticles surface.
The radiation dose enhancement studies with pGNPs in vitro was carried out in HeLa cell lines by clonogenic survival assays (40), which is a more stringent assay for cell viability upon irradiation with X-rays. Colony formation with the HeLa cells treated with nanoparticles was assessed for two weeks post irradiation of the cells with X-rays. Figure 5 shows the number colonies formed in control cells (no pGNPs) and two sets of cells treated with 0.5 and 2 mg/mL of pGNPs. As can be visualized from the plates that non irradiated cells in control as well as pGNPs treated formed approx. ~174 colonies whereas upon irradiation with X-rays, the number of colonies for control cells decreased to 151, 0.5 mg/mL pGNPs to 121 and a mere 42 colonies for the cells treated with 2 mg/mL pGNPs. However, a small decrease in the number of colonies formed for the non-irradiated cells treated with 2 mg/mL pGNPs can be attributed to the high concentration of pGNPs.

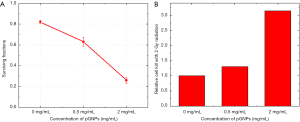
When untreated cells are plated as a single-cell suspension at low densities, they will grow to colonies. PE is the ratio of the number of colonies to the number of cells seeded and act as controls for calculating the SF (40). The PE was calculated for each of the plates, for the control plates without pGNPs and 0.5 mg/mL pGNPs the plating efficiency was ~42% however there was a small decrease in the plating efficiency with 2 mg/mL pGNPs (~36%) which can be attributed to a very high concentration of pGNPs with the cells. However, for calculating the surviving fractions which shows the actual efficacy of the radiation effect on the cells, a correction for the PE was considered for each sample. SF as a function of dose can also be determined by using the clonogenic assay. The radiation dose—survival curve may be modified by the additional treatment, yielding results expressed as a dose-modifying factor, dose enhancement ratio or sensitizer enhancement ratio. As can be visualized from the Figure 6A, for the control samples upon irradiation a SF value of 0.82 was observed. However, for the cells treated with pGNPs, there is almost a linear decrease in the SF value with an increase in the pGNPs concentrations. A SF value of 0.63 and 0.26 was observed for the cells treated with 0.5 and 2 mg/mL respectively. Figure 6B shows the relative cell death upon irradiation in presence of pGNPs when compared with control cells with no pGNPs. It is evident that, with cells treated with 0.5 mg/mL, a relative increase in cell death to 1.3 fold was a result of radiation boost with pGNPs in the form of potent Auger electrons. With an increased concentration of pGNPs the increase in relative cell kill to 2.8 fold clearly demonstrated the radiation dose enhancement with the pGNPs. These results are consistent with our previously published results with 50 nm gold nanoparticles.
Discussion
Gold nanoparticles are known for the radiation dose enhancement because of its high Z number, however for successful translation of these nanoparticles to clinics, the design of the nanoplatform containing the gold nanoparticles becomes very critical. Here we have carefully designed the size of the nanoparticles for maximal tumor uptake with minimal uptake in the RES system yet these long circulating nanoparticles should be cleared via the renal excretion upon disintegration of the surface ligands.
Keeping these parameters in mind we have synthesized third generation of ultrasmall gold nanoparticles of 2-3 nm diameter. The excellent capping properties of the THPC were exploited for in situ reduction of chloroauric acid simultaneously with THPC. The size of the nanoparticles plays a significant role in various physiological parameters (hepatic filtration, renal excretion, tissue extravasation and diffusion) (22,42,43). Bigger size nanoparticles tend to accumulate in RES organs primarily in liver and spleen (25,44). The correlation between the decrease in the size of the nanoparticle with the increase in the nanoparticle’s capacity to navigate between the tumor interstitium after extravasation has been well documented. The hydrodynamic size of the pGNPs formulation reported here is 12 nm which is ideal as this will avoid the faster renal excretion with lower hepatic infiltration and long circulation because of the amphiphilic PEG on the surface. The presence of PEG also imparted excellent long term bench stability to the pGNPs as shown in the stability data. The passive layer of PEG on nanoparticles surface reduces interparticle interactions thereby reducing agglomeration over time.
The small core size of the nanoparticles was stabilized with non immunogenic and biocompatible hetero-bifunctional PEG molecules. The presence of PEG on the surface of nanoparticles not only provides a passive layer but also modulates the surface charge of the nanoparticles which ultimately determines the biodistribution of the nanoparticles. For the pGNPs formulation the surface charge can be modulated easily by varying the ratios of the PEG molecules functionalized with methoxy, amine or carboxyl groups. Gbadamosi et al. have shown a linear correlation between the surface charge of the nanoparticles and the extent of phagocytosis by the macrophage cells (45). Also, the presence of PEG on the nanoparticles surface substantially reduce nonspecific interactions with proteins through its hydrophilicity and steric repulsion effects thereby reducing opsonization (46,47). Furthermore, depending upon the size of the nanoparticles, PEG with a molecular weight of 2-10 kDa has been optimized for an efficient tumor accumulation and minimal phagocytosis (26,48).
The presence of functional groups on the surface of nanoparticles imparts the flexibility to conjugate various biomolecules, fluorophores, drugs or radiolabels. The stabilization of the pGNPs heterobifuntional PEG provided free –NH2 and –COOH groups, out of which –NH2 groups were used for conjugating the AF647. The free-COOH groups can be further conjugated to a peptide or aptamer in order to increase the specific targeting of the nanoparticles to particular organ. The presence of AF647 with pGNPs provides a tool for optically tracking the nanoparticles fate in the systemic circulation. In vitro confocal imaging with the HeLa cells demonstrated the ease and feasibility of the use of these nanoparticles in optical tracking. Also, the biocompatibility of the AF647-pGNPs formulation even at high doses with the HeLa cells shows that this formulation is suitable for in vitro cell labeling, cell tracking, and other bioimaging applications.
The clonogenic survival assay clearly demonstrated the application of the pGNPs platform in enhancing the radiation dose. The results obtained with the pGNPs are very similar to the earlier reports with the same dose rate with HeLa cell line (9,49,50). A 2.8 fold increase in relative cell kill with 2 mg/mL of pGNPs shows the efficiency of this platform in boosting radiation dose. This is important because while fabricating a platform if some of the properties, like generation of Auger electrons in this case, can be hindered due to change in size, surface properties or conjugation to biomolecules, which may result in non-functional nanoparticles with no application. However, the overall data suggests that the platform of ultrasmall AF647-pGNPs has the potential in terms of optical tracking in vitro and boosting the radiation therapeutic effect. The parameters which were used to fabricate this platform clearly shows advantages over other existing formulations of gold nanoparticles for radiation therapy. Such information will be crucial in further application of these nanoparticles in in vivo experiments and further clinical trial design of radiation therapy using these theranostic pGNPs nanoparticles.
Conclusions
We have demonstrated the synthesis of highly monodispersed and ultrafine gold nanoparticles with biocompatible and non-immunogenic PEG on the surface. The size of the nanoparticles has been successfully maintained around 12 nm to prevent quick excretion via the renal route or accumulation in the RES system and hence to enhance tumor accumulation. The pGNPs were conjugated with the AF647 so that the nanoparticles can be tracked in vitro by optical imaging. These nanoparticles are robustly uptaken by cells in culture, and they subsequently exert potent therapeutic effect enhancing the radiation dose as demonstrated by clonogenic survival assays. The small size of the nanoparticles (for long circulation and high half life in circulation), presence of free functionalities on the surface (for conjugating multiple biomolecules like RGD peptide, aptamers), inherent imaging modality (AF647 fluorophore) with excellent radiosensitizing properties makes these AuRadTM platform ideally suitable for further in vivo radiation therapeutic investigations and future clinical translation.
Acknowledgments
Funding: This work was supported partially by NSF-DGE-0965843, HHS/1U54CA151881 CORE1, 1R03 CA164645-01 and a seed grant from the BWH Biomedical Research Institute.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Rao V. L. Papineni, Pataje G.S. Prasanna, Mansoor M. Ahmed) for the series “Nanotechnology in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.07.02). The series “Nanotechnology in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki. Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 2004;104:293-346. [PubMed]
- Badwaik VD, Bartonojo JJ, Evans JW, et al. Single-step biofriendly synthesis of surface modifiable, near-spherical gold nanoparticles for applications in biological detection and catalysis. Langmuir 2011;27:5549-54. [PubMed]
- Mandal S, Bakeine GJ, Krol S, et al. Design, development and characterization of multi-functionalized gold nanoparticles for biodetection and targeted boron delivery in BNCT applications. Appl Radiat Isot 2011;69:1692-7. [PubMed]
- Chang CC, Yang KH, Liu YC, et al. New pathway to prepare gold nanoparticles and their applications in catalysis and surface-enhanced Raman scattering. Colloids Surf B Biointerfaces 2012;93:169-73. [PubMed]
- El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett 2005;5:829-34. [PubMed]
- Lee KY, Hwang J, Lee YW, et al. One-step synthesis of gold nanoparticles using azacryptand and their applications in SERS and catalysis. J Colloid Interface Sci 2007;316:476-81. [PubMed]
- He H, Xie C, Ren J. Nonbleaching fluorescence of gold nanoparticles and its applications in cancer cell imaging. Anal Chem 2008;80:5951-7. [PubMed]
- Melancon MP, Lu W, Yang Z, et al. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol Cancer Ther 2008;7:1730-9. [PubMed]
- Chithrani DB, Jelveh S, Jalali F, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat Res 2010;173:719-28. [PubMed]
- Hainfeld JF, Dilmanian FA, Zhong Z, et al. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys Med Biol 2010;55:3045-59. [PubMed]
- Azzazy HM, Mansour MM, Samir TM, et al. Gold nanoparticles in the clinical laboratory: principles of preparation and applications. Clin Chem Lab Med 2011;50:193-209. [PubMed]
- Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev 2012;41:2256-82. [PubMed]
- Herold DM, Das IJ, Stobbe CC, et al. Gold microspheres: a selective technique for producing biologically effective dose enhancement. Int J Radiat Biol 2000;76:1357-64. [PubMed]
- Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 2004;49:N309-15.
- Berbeco RI, Ngwa W, Makrigiorgos GM. Localized dose enhancement to tumor blood vessel endothelial cells via megavoltage X-rays and targeted gold nanoparticles: new potential for external beam radiotherapy. Int J Radiat Oncol Biol Phys 2011;81:270-6. [PubMed]
- Larson D, Bodell WJ, Ling C, et al. Auger electron contribution to bromodeoxyuridine cellular radiosensitization. Int J Radiat Oncol Biol Phys 1989;16:171-6. [PubMed]
- Hainfeld JF, Dilmanian FA, Slatkin DN, et al. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol 2008;60:977-85. [PubMed]
- Howell RW. Auger processes in the 21st century. Int J Radiat Biol 2008;84:959-75. [PubMed]
- Rahman WN, Wong CJ, Ackerly T, et al. Polymer gels impregnated with gold nanoparticles implemented for measurements of radiation dose enhancement in synchrotron and conventional radiotherapy type beams. Australas Phys Eng Sci Med 2012;35:301-9. [PubMed]
- Chattopadhyay N, Cai Z, Pignol JP, et al. Design and characterization of HER-2-targeted gold nanoparticles for enhanced X-radiation treatment of locally advanced breast cancer. Mol Pharm 2010;7:2194-206. [PubMed]
- Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 2012;14:1-16. [PubMed]
- Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol 2007;25:1165-70. [PubMed]
- Zhang XD, Wu D, Shen X, et al. In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials 2012;33:4628-38. [PubMed]
- Burns AA, Vider J, Ow H, et al. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett 2009;9:442-8. [PubMed]
- Kumar R, Roy I, Ohulchanskky TY, et al. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano 2010;4:699-708. [PubMed]
- Perrault SD, Chan WC. In vivo assembly of nanoparticle components to improve targeted cancer imaging. Proc Natl Acad Sci U S A 2010;107:11194-9. [PubMed]
- Zhang XD, Wu D, Shen X, et al. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012;33:6408-19. [PubMed]
- Liu Y, Ai K, Lu L. Nanoparticulate X-ray computed tomography contrast agents: from design validation to in vivo applications. Acc Chem Res 2012;45:1817-27. [PubMed]
- Burda C, Chen X, Narayanan R, et al. Chemistry and properties of nanocrystals of different shapes. Chem Rev 2005;105:1025-102. [PubMed]
- Hauck TS, Ghazani AA, Chan WC. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small 2008;4:153-9. [PubMed]
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005;26:3995-4021. [PubMed]
- Zhang G, Yang Z, Lu W, et al. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials 2009;30:1928-36. [PubMed]
- Shmeeda H, Tzemach D, Mak L, et al. Her2-targeted pegylated liposomal doxorubicin: retention of target-specific binding and cytotoxicity after in vivo passage. J Control Release 2009;136:155-60. [PubMed]
- Choi CH, Alabi CA, Webster P, et al. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci U S A 2010;107:1235-40. [PubMed]
- Grieneisen ML, Zhang M. Nanoscience and nanotechnology: evolving definitions and growing footprint on the scientific landscape. Small 2011;7:2836-9. [PubMed]
- Ngwa W, Korideck H, Kassis AI, et al. In vitro radiosensitization by gold nanoparticles during continuous low-dose-rate gamma irradiation with I-125 brachytherapy seeds. Nanomedicine 2013;9:25-7. [PubMed]
- Berbeco RI, Korideck H, Ngwa W, et al. DNA damage enhancement from gold nanoparticles for clinical MV photon beams. Radiat Res 2012;178:604-8. [PubMed]
- Duff DG, Baiker A, Edwards PP. A New Hydrosol of Gold Clusters. J Chem Soc Chem Comm 1993;1:96-8.
- Wong J, Armour E, Kazanzides P, et al. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys 2008;71:1591-9. [PubMed]
- Franken NA, Rodermond HM, Stap J, et al. Clonogenic assay of cells in vitro. Nat Protoc 2006;1:2315-9. [PubMed]
- Hu M, Chen J, Li ZY, et al. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem Soc Rev 2006;35:1084-94. [PubMed]
- Fischer HC, Liu L, Pang KS, et al. Pharmacokinetics of nanoscale quantum dots: In vivo distribution, sequestration, and clearance in the rat. Adv Funct Mater 2006;16:1299-305.
- Jain TK, Reddy MK, Morales MA, et al. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm 2008;5:316-27. [PubMed]
- Schipper ML, Nakayama-Ratchford N, Davis CR, et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol 2008;3:216-21. [PubMed]
- Gbadamosi JK, Hunter AC, Moghimi SM. PEGylation of microspheres generates a heterogeneous population of particles with differential surface characteristics and biological performance. FEBS Lett 2002;532:338-44. [PubMed]
- Bazile D, Prud’homme C, Bassoullet MT, et al. Stealth Me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci 1995;84:493-8. [PubMed]
- Li Y, Pei Y, Zhang X, et al. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. J Control Release 2001;71:203-11. [PubMed]
- Larsen EK, Nielsen T, Wittenborn T, et al. Accumulation of magnetic iron oxide nanoparticles coated with variably sized polyethylene glycol in murine tumors. Nanoscale 2012;4:2352-61. [PubMed]
- Kanno I, Fukushi K, Yamaguchi J, et al. Effects of x-ray radiation on HeLa cells. J Radiat Res 1965;6:82-95. [PubMed]
- Rahman WN, Bishara N, Ackerly T, et al. Enhancement of radiation effects by gold nanoparticles for superficial radiation therapy. Nanomedicine 2009;5:136-42. [PubMed]