
Hematopoietic stem cell senescence and cancer therapy-induced long-term bone marrow injury
Introduction
Normal tissue injury remains a serious concern of cancer treatment with ionizing radiation (IR) and/or chemotherapy (1,2). Acute normal tissue injury can lead to dose reductions and delays in therapy. Dose reductions and treatment delays can compromise the outcomes of cancer therapy and decrease overall survival and disease-free survival. In addition, due to an improvement in early detection and treatment of cancer, the number of cancer survivors in the population is increasing. Currently, there are more than 11 million cancer survivors in the United States and most of them are and will be alive five years or more after cancer diagnosis. Unfortunately, long-term cancer survivors are at increasing risks to develop cancer treatment-related late effects that can adversely affect the quality of life, contribute to the ongoing burden of illness and costs, and decrease length of survival. One of the common cancer treatment-related late effects is long-term bone marrow (LT-BM) injury resulting from IR- and/or chemotherapy-induced damage to hematopoietic stem cells (HSCs) (2). The goal of this review is to provide a survey of some of these recent findings regarding the underlying mechanisms by which IR and chemotherapy cause HSC damage, which may facilitate the research to develop new therapeutic strategies to prevent and mitigate the damage and to reduce the long-term effects of conventional cancer therapy on the hematopoietic system.
The hierarchy of the hematopoietic system and HSC niche
The hematopoietic system is organized in a hierarchical manner (3,4). The hierarchical structure of the system has been extensively studied in mice that are widely used as a model system to study IR- and chemotherapy-induced BM injury (Figure 1). The murine hematopoietic system consists of the rare HSCs (CD150+CD48–Lin–Sca1+c-kit+ or CD150+CD48–LSK cells), including long-term HSCs (LT-HSCs, CD34–CD150+CD48–LSK cells) and short-term HSCs (ST-HSCs, CD34+CD150+CD48–LSK cells), at the top of the hierarchy (5,6). They have the ability to self-renew, proliferate, and differentiate into different lineages of peripheral blood cells through multipotent progenitors (MPPs, CD150+/–CD48+LSK cells). HSCs are responsible for sustaining hematopoietic homeostasis and regeneration after injury for the entire life of an organism. In contrast, MPPs and hematopoietic progenitor cells (HPCs) are rapidly proliferating cells with limited and no self-renewal ability, respectively (4). The proliferation and differentiation of MPPs and HPCs not only allow the hematopoietic system to replenish various blood cells on a daily basis during normal hematopoiesis but also enable the system to react swiftly to meet the demand for an increased output of mature cells during hematopoietic crisis, such as loss of blood, hemolysis, or infection.
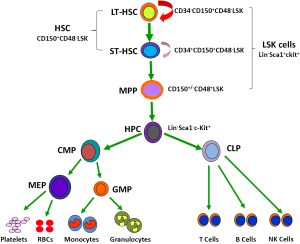
The majority of HSCs are quiescent under a hemostatic condition (5,7,8). These quiescent HSCs normally reside in the osteoblastic niche, which is adjacent to the endosteal bone surface (9-13). The osteoblastic niche provides HSCs with a special environment that supports their self-renewal. This is likely achieved in part by extensive interactions between HSCs and the niche via a variety of soluble factors, such as Wnt (14), bone morphogenetic proteins (15), thrombopoietin (16), interleukin 3 (IL-3), and IL-6 (17); various adhesion molecules, including CXCL12-CXCR4 and N-cadherin (18); and different signaling pathways, for example, stem cell factor/c-Kit, Jagged/Notch, and angiopoietin-1/Tie2 (Ang-1/Tie2) (19). These intricate interactions promote HSC self-renewal in part by keeping them quiescent, because quiescent HSCs have low metabolic activities and thus produce less reactive oxygen species (ROS) that are capable of causing oxidative damage to HSCs (20). In addition, most endosteal osteoblastic niches are considered hypoxic, as they are relatively remote from blood flow (21). It is estimated that the concentration of oxygen in these niches is below 1%. In the mouse BM, HSCs show lower blood perfusion as determined by low Hoechst 33342 (Hoe) staining after the dye injection (22). In addition, HSCs can be enriched in the BM cell populations with the most pimonidazole staining after administration of pimonidazole, a chemical marker for hypoxia (22). Furthermore, human cord blood CD34+ cells became hypoxic and quiescent within a few weeks after transplantation into nonobese diabetic/severe combined immunodeficient interleukin-2 receptor γ chain knockout mice (23). These findings suggest that quiescent HSCs likely reside in a hypoxic environment in the BM. To cope with hypoxia, HSCs express higher levels of hypoxia inducible factor 1α (HIF-1α) (20,24,25). Increased expression of HIF-1α alters the metabolism of HSCs by upregulating glycolysis while downregulating mitochondrial oxidative phosphorylation, leading to reduced production of ROS (25). Therefore, HSCs are presumably better protected from oxidative stress to maintain their ability to self-renew by residing in a hypoxic environment and being a quiescent state. However, during hematopoietic stress HSCs can be mobilized to the vascular niche made up by the sinusoidal endothelial cells (SECs) and several other types of cells in the BM and other hematopoietic tissues (26-28), where they can undergo rapid proliferation and differentiation to generate various hematopoietic cells. Therefore, HSCs may use either osteoblasts or endothelial cells as their niche under different circumstances to maintain a fine balance between quiescence and proliferation or self-renewal and differentiation, as well as to respond to stress.
IR- and chemotherapy-induced BM injury
BM injury is one of the most common dose-limiting side effects of conventional cancer therapy. Acute BM injury occurs shortly after chemotherapy and/or IR due to induction of hematopoietic cell apoptosis (29-32). Because the majority of HSCs are quiescent and more proficient in repairing DNA damage, they are more resistant to induction of apoptosis after exposure to IR and chemotherapy than proliferating MPPs and HPCs (33-35). Therefore, the acute BM injury has been primarily attributed to the induction of apoptosis in the rapidly proliferating MPP and HPC populations by IR and chemotherapy (1,36). Under this circumstance, HSCs undergo self-renewing proliferation and differentiation to repopulate MPPs and HPCs that in turn generate mature blood cells to restore the homeostasis of the hematopoietic system. Since HSC, MPP and HPC proliferation and differentiation can be stimulated by various hematopoietic growth factors (HGFs) such as granulocyte-colony stimulating factor (G-CSF), granulocyte/macrophage-colony stimulating factor (GM-CSF) or erythropoietin, these HGFs have been widely used in clinic to promote the recovery of BM hematopoietic function in patients after cancer therapy (37-39). As such, the majority of cancer patients can recover rapidly from acute BM suppression after chemotherapy and/or IR with or without HGF treatment.
However, some patients can develop LT-BM injury after chemotherapy and/or IR due to HSC damage (2). The occurrence of LT-BM injury is more prevalent both in patients and experimental animals receiving treatment with carboplatinum, busulfan, bis-chloronitrosourea (BCNU) and/or exposed to a moderate or a high dose of total body irradiation (TBI) (40-42). Unlike acute BM suppression, LT-BM damage is latent. Patients and animals with LT-BM injury usually have normal blood cell counts under normal homeostatic conditions in spite of a decrease in HSC reserves and an impairment in HSC self-renewal (1,2). Because of this latency, the clinical implications of LT-BM injury have been largely overlooked. Moreover, the importance of LT-BM damage is further obscured by the seemingly complete recovery of peripheral blood cell counts, BM cellularity and the number of colony-forming units (CFUs), especially after the use of HGFs. In fact, the use of HGFs may worsen chemotherapy- and IR-induced LT-BM damage by promoting HSC, MPP and HPC proliferation and differentiation at the expense of HSC self-renewal (37,38,43). This could lead to an accelerated exhaustion of HSCs and further compromise the long-term recovery of BM hematopoietic function. Although LT-BM damage is latent, it is long lasting and shows little tendency for recovery, and can lead to the development of hypoplastic anemia or a myelodysplastic syndrome at later times or following additional hematopoietic stress such as subsequent cycles of consolidation cancer treatment or BM transplantation.
HSC senescence—an underlying mechanism whereby IR and chemotherapy induce LT-BM injury
Several mechanisms have been proposed to explain how IR as well as some chemotherapeutic agents induces LT-BM injury, including: (I) induction HSC apoptosis and/or differentiation; (II) induction of HSC senescence; and/or (III) damage to BM stromal cells or the HSC niche (29,32,42,44). Although induction of HSC apoptosis and differentiation can contribute to IR- and chemotherapy-induced acute BM injury, it may play an insignificant role in LT-BM damage, because HSC can undergo self-renewing proliferation to repopulate the depleted HSCs if the ability of HSC to self-renew is not impaired by IR and chemotherapy. In addition, endosteal osteoblasts, a major component of the osteoblastic niche, are relatively resistant to IR and chemotherapy and are capable of supporting donor cell engraftment after myeloablation and HSC transplantation (45). In fact, it was shown recently that after BM radioablation, endosteal osteoblasts underwent rapid expansion in response to megakaryocyte-derived mesenchymal growth factors such as platelet-derived growth factor-β and basic fibroblast growth factor to promote HSC engraftment and hematopoietic reconstitution after BM transplantation by restoring the damaged HSC niche (46). However, it has been shown recently that exposure to IR induces BM stromal cell senescence (44). It remains to be determined whether induction of BM stromal cell senescence and damage to other cells in the HSC niche also affect HSC self-renewal and contribute to IR- and chemotherapy-induced LT-BM injury. Compared to various BM stromal cells and other hematopoietic cells, HSCs are relatively more sensitive to oxidative stress, probably in part because they normally reside in a hypoxic environment in the HSC niche and maintain in a quiescent state. Therefore, a moderate increase in ROS is capable of impairing the ability of HSCs to self-renew via induction of HSC senescence, which can cause premature exhaustion of HSCs and LT-BM suppression (47-49). Therefore, induction of HSC senescence resulting from increased production of ROS has been implicated in the pathogenesis of BM suppression under various pathological conditions, including LT-BM injury induced by IR and chemotherapy (47-49). The first evidence that HSCs can undergo senescence was observed in Bmi1-/- mice. It was found that mice lacking the Bmi1 gene developed progressive BM hypoplasia and died early (<2 months) after birth (50,51). Although Bmi1-/- mice had a normal pool of fetal liver HSCs, transplantation of their fetal liver HSCs to a lethally irradiated recipient resulted only in a transient reconstitution of the hematopoietic system (50,51). This suggests that the mutant fetal liver HSCs have the ability to proliferate and differentiate into HPCs enabling transient reconstitution of the BM, but cannot self-renew and generate HSCs to ensure long-term hematopoietic engraftment. Deficiency in self-renewal was also found in neural and leukemia stem cells lacking Bmi1, indicating that Bmi1 is a general regulator of stem cell self-renewal (50-52). Bmi1 is a member of the Polycomb group of transcriptional repressors. Its downstream targets include the gene products of the Ink4a/Arf locus, e.g., p16Ink4a (p16) and Arf. HSCs from Bmi1-/- mice express increased levels of p16 and Arf (50-52). Enforced expression of p16 and Arf in HSCs induces cell cycle arrest and apoptosis, respectively, whereas p16 knockout partially restores the ability of Bmi1-/- stem cells to self-renew (50-52).
Similarly, it has been hypothesized that IR and chemotherapy cause LT-BM injury primarily by induction of HSC senescence which impairs HSC replication and self-renewal leading to the reduction in HSC reserves (53-55). Impairment in HSC self-renewal has been well documented in patients and animals after exposure to TBI or treatment with various chemotherapeutic agents that can cause LT-BM injury (1,55,56). For example, BM HSCs from mice after exposure to IR or receiving chemotherapy generated fewer colony-forming unit-spleen (CFU-S) and repopulating units in lethally irradiated recipients after BM transplantation (38,57-60). Similar impairments of HSC self-renewal capacity and long-term repopulating ability were observed in patients undergoing autologous transplantation after TBI and/or dose-intensified chemotherapy. However, direct evidence to demonstrate that HSCs undergo senescence after exposure to IR or a chemotherapeutic agent was lacking until our recent studies. In these studies, we found that exposure to IR or busulfan treatment induced HSC senescence in vitro and in vivo (41,42,55,61). The senescent HSCs induced by IR and busulfan had diminished clonogenic activity and expressed increased levels of SA-β-gal, p16, and Arf. Interestingly, a shortening of the intrinsic replicative capacity of HSCs or loss of HSC self-renewal after exposure to IR does not affect HSC differentiation to generate various HPCs and more mature progeny prior to their final exhaustion. Moreover, HPCs from irradiated mice showed neither abnormalities nor did they exhibit signs of senescence. These findings indicate that IR can selectively induce HSC senescence (42,55).
ROS and HSC senescence
ROS is a collective term for oxygen species that are more reactive than oxygen molecule. The common ROS in a biologic system includes hydrogen peroxide, superoxide, and hydroxyl radical. Hydroxyl radical and superoxide also are free radicals that have an unpaired valence electron. Hydroxyl radical is highly reactive and short lived. It can react to a variety of macromolecules to cause oxidative damage to a cell and thus is highly toxic. Superoxide can be spontaneously converted into hydrogen peroxide or rapidly dismutated to hydrogen peroxide by superoxide dismutase (SOD). Hydrogen peroxide is less reactive than superoxide and thus biologically less toxic. It can be converted into highly toxic hydroxyl radicals through acquisition of an electron or eliminated by catalase, glutathione peroxidase (GPX), or peroxiredoxin. Because hydrogen peroxide is less reactive with a longer half-life and membrane permeable, it can also function as a signal molecule to regulate various biological activities in a cell.
ROS can regulate HSC function in a concentration-dependent manner. Low levels of ROS appear to be required for HSC proliferation, differentiation, and mobilization (62-65). For example, it was reported recently that HSCs from AKT1/2 double knockout mice exhibit a defect in long-term hematopoietic reconstitution after transplantation (62). The defect is attributable to the reduced production of ROS, as moderate elevation of ROS in HSCs by incubation of the cells from the knockout mice with low doses of the pro-oxidant L-buthionine-S,R-sulfoximine (BSO) increased their clonogenicity. This is in agreement with another recent observation that ROS-dependent proliferation of HSCs also plays an important role in the early steps of hematopoietic reconstitution after HSC transplantation (65).
However, increased production of ROS can be detrimental to HSCs (61,66-73). For example, incubation of HSCs with BSO, which induces oxidative stress by depleting intracellular reduced glutathione, resulted in a dramatic reduction in HSC clonogenicity (62,74). A moderate increase in ROS production after deletion of the ataxia telangiectasia mutated gene (ATM) can disrupt HSC quiescence by stimulating HSC cycling, which comprises the ability of HSCs to self-renewal and leads to HSC premature exhaustion (67,75). Treatment of ATM-/- mice with N-acetyl-cysteine can restore the function of HSCs and prevent the development of BM failure (75). Subsequently, it was shown that the number of HSCs and their long-term repopulating activity were markedly reduced in association with an increased production of ROS in HSCs after the deletion of the genes encoding the O subclass of the forkhead family of transcription factors, e.g., FoxOs (FoxO1, FoxO3, and FoxO4) in mice (68). These defects were associated with an increased production of ROS in HSCs and ameliorated by the treatment with N-acetylcysteine (NAC). In addition, increased production of ROS is also association with HSC defect in several other pathological conditions, including deletion of Bmi1 (71,76), the Mouse double minute 2 homolog gene (MDM2) (69) and the tuberous sclerosis 1 gene (TSC1) (70), Fanconi anemia mutation (77), and aging (66,78-81).
It has been well established that exposure of a cell to IR or treatment with certain chemotherapeutic agents cause immediate increase in ROS production. This initial oxidative stress not only produces direct and acute cell damage, but more importantly also perturbs cellular metabolism to disturb the balance of reduction/oxidation (redox) reactions, leading to a persistent and prolonged increase in ROS production (82). The induction of chronic oxidative stress by IR and chemotherapy sets in motion a self-perpetuating process of late deleterious effects, including cellular senescence, genetic instability, inflammation and fibrosis (61,82,83). This new concept is supported by the observations that: (I) an increase in the formation of oxidized products persists in various tissues even days, weeks and months after chemotherapy or exposure to IR; (II) a non-targeted effect of IR can occur in cells that are not themselves irradiated but are the descendants of irradiated cells (irradiation-induced genomic instability) or in non-irradiated cells that are in contact with irradiated cells (radiation-induced bystander effects); and (III) a post-irradiation treatment with an antioxidant can ameliorate some of the late detrimental effects induced by IR (55,61,83). Therefore, chemotherapy- and IR-induced late effects that once were viewed as an inevitable and untreatable consequence of the initial and direct insult of chemotherapy and IR, are now considered as dynamic responses of the cells affected by the insult and thus, amendable to therapeutic intervention. This hypothesis has been extensively examined in several organs that are sensitive to chemotherapy- and IR-induced late tissue injury, such as fibrotic injury in the lungs and kidneys (84,85). However, evidence to support the hypothesis that induction of chronic oxidative stress may play an important role in mediating IR- and chemotherapy-induced late effects on the hematopoietic system was lacking until our recent studies. In these studies, we found that exposure of mice to a sublethal dose of TBI induced a persistent increase in ROS production only in HSCs, but not in their progeny (61). The induction of chronic oxidative stress was associated with sustained increases in oxidative DNA damage and DNA double strand breaks (DSBs) in HSCs, decreases in HSC clonogenic function and long-term engraftment ability, and myeloid skewing (55,61,75,86). Treatment of irradiated mice with an antioxidant such as NAC or the SOD mimetic MnTE-2-PyP after TBI significantly attenuated IR-induced LT-BM injury (49,55,61). These findings provide the first evidence that TBI causes LT-BM damage via induction of chronic oxidative stress selectively in HSCs.
Role of the p38 mitogen-activated protein kinase (p38)-p16 pathway in regulation of HSC senescence
Although an acute and excessive production of ROS occuring shortly after exposure to a high dose of IR can cause apoptosis in a variety of cells including HSCs via induction of DSBs and activation of the p53 pathway (29), chronic oxidative stress (61) induced by chemotherapy and IR appears to injury HSCs not by a nonspecific cytotoxic effect as previously hypothesized but via induction of cellular senescence at least in part through redox-dependent activation of the p38-p16 pathway (66).
p38 is a member of the mitogen-activated protein kinase (MAPK) family of signal transduction kinases, which can be activated in a sequential order [mitogen-activated or extracellular signal-regulated kinase kinase (MEKK)-MAPK kinase 3/6 (MKK3/6)-p38] after exposure to stress (87). Activation of p38 regulates a variety of cellular processes such as inflammation, cell cycle arrest and apoptosis in a cell type specific manner. It also plays a critical role in the induction of senescence in response to a variety of stimuli via up-regulating p16 (88-90). For example, it was shown that a high level of Ras or Raf activation in human normal fibroblasts induced senescence by stimulating a sustained activation of p38, which in turn upregulated the expression of p16 (89,90). Activation of the p38 pathway also contributes to the induction of p16 and cellular senescence after DNA damage resulting from exposure to genotoxic and oxidative stress and telomere shortening due to extensive replication (91) (Figure 2). Furthermore, activation of p38 by ectopic transfection of MKK3 and/or MKK6 increases p16 expression and induces senescence. In contrast, inhibition of p38 activity or down-regulation of p38 expression attenuates the induction of p16 and cellular senescence by oncogenic stress, DNA damage and telomere shortening (89,91,92).
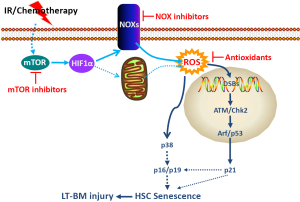
ROS can activate p38 via the apoptosis signal-regulating kinase 1 (ASK1) (93). Normally, ASK-1 forms an inactive complex with the repressor protein thioredoxin in a cell. The formation of this complex is dependent on the presence of a reduced form of an intramolecular disulfide bridge between two cysteine residues of thioredoxin. Oxidation of thioredoxin by ROS causes dissociation of ASK-1 from thioredoxin, resulting in activation of ASK1 by oligomerization, interaction with TNF receptor-associated factor-2/6, and threonine autophosphorylation (93). It has been shown that ROS production from Nox4 can activate p38 via activation of ASK-1 (94). Alternatively, ROS can activate p38 by inactivation of protein tyrosine phosphatases (PTPs) (95,96), because oxidation of the catalytic cysteine of PTPs by ROS can reversibly inactivate PTPs (97).
Activation of p38 has been implicated in BM suppression in various pathological conditions, including aplastic anemia and myelodysplastic syndromes (98,99). Furthermore, recently it was shown that mutation of the ATM gene and knockout of the FoxO3 gene induced premature senescence/exhaustion of HSCs (67,68,72). The induction of HSC senescence/exhaustion was associated with an elevated production of ROS, a selective activation of p38, and an upregulation of p16 in HSCs. Pharmacological inhibition of p38 activity rescued the defects of HSCs from ATM mutants and FoxO3 knockout mice (67,68,72). These findings indicate that p38 plays an important role in regulation of HSC self-renewal and its activation by oxidative stress can mediate the induction of HSC senescence via regulation of p16 (66). Therefore, we recently examined whether IR causes hematopoietic suppression in part by inducing hematopoietic cell senescence through activation of the p38 pathway and whether pharmacological inhibition of p38 can attenuate IR-induced residual BM injury (100-102). In this study, we found that p38 was selectively activated in irradiated hematopoietic cells and this activation sustained up to 5 weeks after IR in a LT-BM cell culture assay. Inhibition of p38 activity with a specific inhibitor, e.g., SB203580, attenuated IR-induced suppression of BM hematopoietic cell function in association with a significant reduction in p16 expression and SA-β-gal activity. Moreover, our in vivo data shows that inhibition of p38 attenuated IR-induced LT-BM suppression. These results suggest that p38 activation plays a role in mediating IR-induced hematopoietic cell senescence and BM suppression and that pharmacological inhibition of the p38 pathway with a specific inhibitor can be further exploited for amelioration of IR-induced residual BM injury (100-102).
The Ink4a-Arf locus encodes two tumor suppressors, p16 and Arf (103-105). The transcripts for these proteins have different first exons (α for p16 and β for Arf), but share exons 2 and 3. However, there is no amino acid sequence similarity between these two proteins due to the use of alternative reading frames for their translation. p16 is a potent cyclin-dependent kinase (CDK) 4/6 inhibitor. By inhibiting CDK4/6 activity, p16 causes Rb hypophosphorylation and suppresses the expression of E2F-dependent genes, resulting in restriction of G1/S cell cycle progression and formation of senescence-associated heterochromatic foci (SAHF) (103,104,106). Once SAHF are formed after the engagement of the p16-Rb pathway, the cells become permanently growth arrested and senescent. It has therefore been suggested that diverse stimuli can induce cellular senescence via various upstream signal transduction cascades, including the p38 and p53-p21 pathways, but converge on the p16-Rb pathway, whose activation provides an inescapable barrier preventing senescent cells from re-entering the cell cycle. This suggestion is supported by the finding that activation of p53 and induction of p21 in cells undergoing senescence are transient events that occur during the onset of senescence and then subside when the expression of p16 starts rising (107-109). Inactivation of p53 prior to upregulation of p16 can prevent senescence induction. However, once p16 is highly expressed, cell cycle arrest becomes irreversible by downregulation of p53, indicating that activation of the p53-p21 pathway plays an important role in the initiation of senescence, but induction of p16 is required for the maintenance of senescence (107,110). In agreement with this suggestion, we found that IR induced p53 activation and p21 expression in HSCs prior to the induction of p16 (41). While p53 activation and p21 upregulation gradually declined within a few weeks after IR, p16 expression in irradiated HSCs remained elevated and the cells subsequently became senescent, exhibiting positive SA-β-gal staining. In contrast, the biological action of Arf relies on the p53 pathway. This is because Arf can directly bind to MDM2 and cause the accumulation of p53 by segregating MDM2 from p53 and by inhibiting MDM2’s E3 ubiquitin protein ligase activity for p53 (103,104,111). Therefore, activation of p53 by Arf can induce not only cellular senescence but also apoptosis, depending on which gene down-stream of p53 is induced following its activation.
Upregulation of p16 and Arf has been implicated in mediating the induction of cellular senescence in a variety of cells including HSCs. For example, increased expression of p16 and Arf was found in HSCs from Bmi1-/- mice (50). However, it appears that p16 but not Arf plays an important role in mediating the induction of Bmi1-/- HSC senescence (50). In addition, it has been found that knockout of both the p16 and Arf genes in mice significantly increases the clonal expansion of HSCs in vitro but modestly promotes HSC self-renewal in vivo (51,112). However, knockout of the Arf gene alone does not provide any advantage for HSC/HPC expansion and self-renewal (113). In contrast, knockout p16 increases the life-span of HSCs by promoting HSC self-renewal (113,114). Furthermore, mutation of the ATM gene also results in up-regulation of p16 and Arf in HSCs (67,115). Inactivation of the p16-Rb pathway by retroviral transfection of human papillomavirus (HPV) E7 proteins restores the reproductive function of ATM-/- HSCs, while inhibition of the Arf-p53 pathway by E6 transfection has no such effect (75). These findings suggest that p16 plays a more significant role than Arf in regulation of HSC self-renewal and induction of HSC senescence, even though both proteins are over-expressed in senescent HSCs. Increased expression of p16 and Arf has been found in IR-induced senescent LSK (Lin-Sca1+c-kit+) cells that are enriched with HSCs (42). However, their roles in mediating IR-induced HSC senescence and LT-BM suppression remain to be investigated.
ROS cellular origines and regulation of ROS production in HSCs
During normal homeostasis, the levels of intracellular ROS in a cell are tightly regulated by a fine balance between ROS production and expression of cellular antioxidant defense enzymes and molecules (116). Therefore, increased production of ROS and/or reduced expression of antioxidant enzymes and molecules can perturb the balance, leading to oxidative stress in HSCs (48,116). For example, ATM has the ability to regulate the activities of several antioxidant enzymes at the levels of transcription and post-translation (117). The cells including HSCs from ATM-/- mice produced increased levels of ROS due to dysregulation of SOD, catalase, and GPX (117). FoxOs can regulate the expression of SOD and catalase at the level of transcription. Knockout of FoxOs in mice induces oxidative stress in HSCs and HSC senescence (68,72). This is mainly attributed to the reduction in SOD and catalase expression (68). In addition, there is a mechanistic link between ATM and FoxO3 in regulation of ROS production in HSCs as FoxO3 is essential for ATM expression (73).
ROS is one of the by-products of mitochondrial respiration, and mitochondria have been considered the main source of cellular-derived ROS in most cells (82). Cells from Bmi1-/- mice produce increased levels of ROS due to impairment in mitochondrial function (71). However, compared to their progeny and other somatic cells, HSCs are dormant, have fewer mitochondria, and primarily utilize glycolysis rather than oxidative phosphorylation for ATP production (118,119). Therefore, HSCs are unique and may depend less on mitochondria for the production of ROS. Recently, an increasing body of evidence demonstrates that cells can also actively produce ROS through a family of tightly regulated NADPH oxidases (NOXs) that are homologues of the phagocyte oxidase (Phox or NOX2) (120,121). ROS produced by NOXs participate in regulation of many cell functions and have been implicated in the pathogenesis of different diseases. HSCs in humans express NOX1, NOX2, and NOX4, as well as various NOX regulatory subunits (118,119). In addition, NOX-mediated extramitochondrial oxygen consumption accounts for an estimated one-half of endogenous cell respiration in human HSCs. These findings suggest that NOXs, but not mitochondria, may be the main cellular origins of ROS in HSCs (118,119). This suggestion is supported by a recent finding showing that IR-induced persistent increase in ROS production in human CD34+ cells has a non-mitochondrial origin (122). More importantly, our recent studies showed that even though HSC-enriched LSK cells from mouse BM expressed NOX1, NOX2, and NOX4, their progeny including BM HPCs, Lin– cells, and monocytes expressed NOX1 and NOX2, but not NOX4, suggesting that NOX4 expression is downregulated upon HSC differentiation and that NOX4 may play an important role in regulation of HSC function (61). More important, it was found that NOX4 expression was upregulated, whereas NOX1 and NOX2 expression was unchanged in HSCs after TBI. Because NOX4 is constitutively active and ROS production by it is regulated at the transcriptional level (120,121,123), the finding that IR upregulates NOX4 in HSCs implies that NOX4 may primarily mediate the IR-induced increase in ROS production in HSCs. This suggestion is supported by the finding that diphenyl iodonium (DPI), but not apocynin, inhibits IR-induced elevation of ROS production in HSCs, because NOX4 is not sensitive to apocynin inhibition, whereas other NOXs are (61,120,121,123). In addition, our recent results showed that resveratrol, a potent antioxidant and a putative activator of Sirtuin 1, could ameliorate TBI-induced LT-BM injury by inhibiting radiation-induced chronic oxidative stress and senescence in HSCs in part by downregulation of NOX4 expression (49). These findings suggest that NOX4 is likely one of the main cellular sources of ROS in HSCs after TBI. However, it has yet to be determined whether other ROS production enzymes (such as cyclooxygenases and lipoxygenases) also play a role in contributing to the increased production of ROS in HSCs induced by IR and chemotherapy.
NOX4 expression is regulated by a variety of factors (124-130). Two of the major factors inducing NOX4 expression, angiotensin II (Ang II) and transforming growth factor-β (TGFβ), have been implicated in mediating various late effects of IR (39,131,132). Both of them may regulate NOX4 expression via activation of the mammalian target of rapamycin (mTOR) because Ang II and TGFβ can activate mTOR via induction of TR3 (or Nur77, a member of the steroid/thyroid/retinoid receptor family) and suppression of DEPTOR (an intracellular mTOR inhibitor) through Smad3, respectively (127-130). mTOR is a serine/threonine kinase that senses and integrates a variety of environmental cues, including nutrients, growth factors, and intracellular energy status, to regulate metabolism and cell growth. It also plays a critical role in regulation of HSC quiescence, self-renewal, and function. Overactivation of mTOR by deletion of Pten or Tsc1 in mice impairs HSC self-renewal and causes HSC exhaustion in association with increased production of ROS (70,133-137). Treatment with rapamycin or NAC can rescue HSC defects in Tsc1 knockout mice, demonstrating that ROS are responsible for mediating mTOR overactivation-induced HSC dysfunction (70). It was hypothesized that hyperactivation of mTOR elevates ROS production by mitochondria because activation of mTOR increases mitochondrial biogenesis (138,139). However, evidence to support this hypothesis has not been presented. In contrast, it was recently shown that mTOR activation increased NOX4 expression and ROS production in epithelial cells (140,141). mTOR may upregulate NOX4 expression via HIF1, because mTOR can activate HIF1 and NOX4 is a new target gene of HIF1 (142-145). These findings suggest that activation of mTOR may increase ROS production primarily via NOX4 rather than mitochondria. Our recent studies showed that mTOR could be activated in HSCs after mice were exposed to a sublethal dose of TBI and that inhibition of mTOR with rapamycin could attenuate TBI-induced increase in ROS production in HSCs (unpublished observation). Therefore, it will be of a great interest to determine whether activation of mTOR mediates IR-induced upregulation of NOX4 in HSCs and whether inhibition of mTOR with an mTOR inhibitor can inhibit IR-induced expression of NOX4 in HSCs. These studies will help identify additional novel molecular targets and therapeutics to inhibit IR-induced HSC senescence and LT-BM injury.
Summary
LT-BM injury is a common cancer treatment-related late effect caused by IR- and chemotherapy-induced damage to HSCs. Although LT-BM damage is latent, it is long lasting, shows little tendency for recovery, and can lead to hypoplastic anemia and predispose individuals treated with IR and chemotherapy to therapy-related myelodysplastic syndrome and acute myeloid leukemia (37,38,53,146). However, neither the mechanisms by which IR and/or chemotherapy induce HSC damage have been clearly defined, nor has an effective treatment been developed to ameliorate LT-BM injury. In this review, we have summarized a number of recent findings regarding the role of oxidative stress in mediating IR- and chemotherapy-induced HSC senescence and LT-BM. These findings provide not only new insights into the molecular mechanisms whereby IR and chemotherapy induce HSC senescence but also potential novel therapeutic strategies that can be exploited to prevent and mitigate IR- and chemotherapy-induced LT-BM injury in cancer patients (Figure 2). Such strategies have the potential to significantly reduce the long-term adverse effects of conventional cancer therapy on the hematopoietic system, increase the compliance of cancer patients to subsequent consolidation cancer treatments, facilitate the long-term engraftment and recovery of hematopoietic function after autologous and allogeneic BM transplantation, and reduce the risk of developing therapy-related myelodysplastic syndrome and acute myeloid leukemia.
Acknowledgments
We apologize to authors whose contributions were not directly cited owing to space limitations. The research conducted in Dr. Zhou’s Laboratory was supported in part by grants from the National Institutes of Health (R01-CA122023 and AI080421) and a grant from the Edward P. Evan’s Foundation, and those in Dr. Meng’s Laboratory by a grant from the National Natural Science Foundation of China (NSFC 30828011).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Stem Cells in Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.10.05). The series “Stem Cells in Cancer” was commissioned by the editorial office without any funding or sponsorship. DZ served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 1995;31:1319-39. [PubMed]
- Testa NG, Hendry JH, Molineux G. Long-term bone marrow damage in experimental systems and in patients after radiation or chemotherapy. Anticancer Res 1985;5:101-10. [PubMed]
- Reya T. Regulation of hematopoietic stem cell self-renewal. Recent Prog Horm Res 2003;58:283-95. [PubMed]
- Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: Origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol 2001;17:387-403. [PubMed]
- Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly Switch from dormancy to self-renewal during homeostasis and repair. Cell 2008;135:1118-29. [PubMed]
- Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005;121:1109-21. [PubMed]
- Foudi A, Hochedlinger K, Van Buren D, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol 2009;27:84-90. [PubMed]
- van der Wath RC, Wilson A, Laurenti E, et al. Estimating dormant and active hematopoietic stem cell kinetics through extensive modeling of bromodeoxyuridine label-retaining cell dynamics. PLoS One 2009;4:e6972 [PubMed]
- Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci 2007;1106:41-53. [PubMed]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol 2005;21:605-31. [PubMed]
- Wilson A, Oser GM, Jaworski M, et al. Dormant and self-renewing hematopoietic stem cells and their niches. Ann N Y Acad Sci 2007;1106:64-75. [PubMed]
- Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003;425:836-41. [PubMed]
- Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003;425:841-6. [PubMed]
- Sugimura R, He XC, Venkatraman A, et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 2012;150:351-65. [PubMed]
- Goldman DC, Bailey AS, Pfaffle DL, et al. BMP4 regulates the hematopoietic stem cell niche. Blood 2009;114:4393-401. [PubMed]
- Yoshihara H, Arai F, Hosokawa K, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 2007;1:685-97. [PubMed]
- Barria E, Mikels A, Haas M. Maintenance and self-renewal of long-term reconstituting hematopoietic stem cells supported by amniotic fluid. Stem Cells Dev 2004;13:548-62. [PubMed]
- Haug JS, He XC, Grindley JC, et al. N-cadherin expression level distinguishes reserved versus primed states of hematopoietic stem cells. Cell Stem Cell 2008;2:367-79. [PubMed]
- Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004;118:149-61. [PubMed]
- Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 2010;7:391-402. [PubMed]
- Winkler IG, Barbier V, Wadley R, et al. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood 2010;116:375-85. [PubMed]
- Parmar K, Mauch P, Vergilio JA, et al. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A 2007;104:5431-6. [PubMed]
- Shima H, Takubo K, Tago N, et al. Acquisition of G0 state by CD34-positive cord blood cells after bone marrow transplantation. Exp Hematol 2010;38:1231-40. [PubMed]
- Du J, Chen Y, Li Q, et al. HIF-1α deletion partially rescues defects of hematopoietic stem cell quiescence caused by Cited2 deficiency. Blood 2012;119:2789-98. [PubMed]
- Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010;7:380-90. [PubMed]
- Yin T, Li L. The stem cell niches in bone. J Clin Invest 2006;116:1195-201. [PubMed]
- Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med 2004;10:64-71. [PubMed]
- Kopp HG, Avecilla ST, Hooper AT, et al. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349-56. [PubMed]
- Shao L, Sun Y, Zhang Z, et al. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood 2010;115:4707-14. [PubMed]
- Lotem J, Sachs L. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood 1993;82:1092-6. [PubMed]
- Wlodarski P, Wasik M, Ratajczak MZ, et al. Role of p53 in hematopoietic recovery after cytotoxic treatment. Blood 1998;91:2998-3006. [PubMed]
- Yu H, Shen H, Yuan Y, et al. Deletion of puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood 2010;115:3472-80. [PubMed]
- Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the Consequences of lethal irradiation. Blood 1998;91:2272-82. [PubMed]
- Meng A, Wang Y, Brown SA, et al. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp Hematol 2003;31:1348-56. [PubMed]
- Mohrin M, Bourke E, Alexander D, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell 2010;7:174-85. [PubMed]
- Dainiak N. Hematologic Consequences of exposure to ionizing radiation. Exp Hematol 2002;30:513-28. [PubMed]
- Gardner RV, Begue R, Mckinnon E. The effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on primitive hematopoietic stem cell (PHSC) function and numbers, after chemotherapy. Exp Hematol 2001;29:1053-9. [PubMed]
- van Os R, Robinson S, Sheridan T, et al. Granulocyte colony-stimulating factor enhances bone marrow stem cell damage caused by repeated administration of cytotoxic agents. Blood 1998;92:1950-6. [PubMed]
- Pineda JR, Daynac M, Chicheportiche A, et al. Vascular-derived TGF-β increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain. EMBO Mol Med 2013;5:548-62. [PubMed]
- Santos GW. Preparative regimens: chemotherapy versus chemoradiotherapy. A historical perspective. Ann N Y Acad Sci 1995;770:1-7. [PubMed]
- Meng A, Wang Y, Van Zant G, et al. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res 2003;63:5414-9. [PubMed]
- Wang Y, Schulte BA, Larue AC, et al. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006;107:358-66. [PubMed]
- van Os R, Robinson S, Sheridan T, et al. Granulocyte-colony stimulating factor impedes recovery from damage caused by cytotoxic agents through increased differentiation at the expense of self-renewal. Stem Cells 2000;18:120-7. [PubMed]
- Carbonneau CL, Despars G, Rojas-Sutterlin S, et al. Ionizing radiation-induced expression of INK4a/ARF in murine bone marrow-derived stromal cell populations interferes with bone marrow homeostasis. Blood 2012;119:717-26. [PubMed]
- Sugrue T, Lowndes NF, Ceredig R. Mesenchymal stromal cells: radio-resistant members of the bone marrow. Immunol Cell Biol 2013;91:5-11. [PubMed]
- Olson TS, Caselli A, Otsuru S, et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 2013;121:5238-49. [PubMed]
- Diaz-Montero CM, Wang Y, Shao L, et al. The glutathione disulfide mimetic NOV-002 inhibits cyclophosphamide-induced hematopoietic and immune suppression by reducing oxidative stress. Free Radic Biol Med 2012;52:1560-8. [PubMed]
- Shao L, Li H, Pazhanisamy SK, et al. Reactive Oxygen species and hematopoietic stem cell senescence. Int J Hematol 2011;94:24-32. [PubMed]
- Zhang H, Zhai Z, Wang Y, et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med 2013;54:40-50. [PubMed]
- Park IK, Qian D, Kiel M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 2003;423:302-5. [PubMed]
- Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 2003;423:255-60. [PubMed]
- Molofsky AV, He S, Bydon M, et al. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev 2005;19:1432-7. [PubMed]
- Fleenor CJ, Marusyk A, Degregori J. Ionizing radiation and hematopoietic malignancies: altering the adaptive landscape. Cell Cycle 2010;9:3005-11. [PubMed]
- Marusyk A, Casás-Selves M, Henry CJ, et al. Irradiation alters selection for oncogenic mutations in hematopoietic progenitors. Cancer Res 2009;69:7262-9. [PubMed]
- Li H, Wang Y, Pazhanisamy SK, et al. Mn(III) meso-tetrakis-(N-ethylpyridinium-2-yl) porphyrin mitigates total body irradiation-induced long-term bone marrow suppression. Free Radic Biol Med 2011;51:30-7. [PubMed]
- von Wangenheim KH, Peterson HP, Feinendegen LE. Residual radiation effect in the murine hematopoietic stem cell compartment. Radiat Environ Biophys 1986;25:93-106. [PubMed]
- Spangrude GJ, Brooks DM, Tumas DB. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: in vivo expansion of stem cell phenotype but not function. Blood 1995;85:1006-16. [PubMed]
- Gardner RV, Lerner C, Astle CM, et al. Assessing permanent damage to primitive hematopoietic stem cells after chemotherapy using the competitive repopulation assay. Cancer Chemother Pharmacol 1993;32:450-4. [PubMed]
- Mauch P, Rosenblatt M, Hellman S. Permanent loss in stem cell self renewal capacity following stress to the marrow. Blood 1988;72:1193-6. [PubMed]
- Neben S, Hellman S, Montgomery M, et al. Hematopoietic stem cell deficit of transplanted bone marrow previously exposed to cytotoxic agents. Exp Hematol 1993;21:156-62. [PubMed]
- Wang Y, Liu L, Pazhanisamy SK, et al. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med 2010;48:348-56. [PubMed]
- Juntilla MM, Patil VD, Calamito M, et al. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive Oxygen species. Blood 2010;115:4030-8. [PubMed]
- Kinder M, Wei C, Shelat SG, et al. Hematopoietic stem cell function requires 12/15-lipoxygenase-dependent fatty acid metabolism. Blood 2010;115:5012-22. [PubMed]
- Owusu-Ansah E, Banerjee U. Reactive Oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 2009;461:537-41. [PubMed]
- Lewandowski D, Barroca V, Ducongé F, et al. In vivo cellular imaging pinpoints the role of reactive Oxygen species in the early steps of adult hematopoietic reconstitution. Blood 2010;115:443-52. [PubMed]
- Ito K, Hirao A, Arai F, et al. Reactive Oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 2006;12:446-51. [PubMed]
- Ito K, Takubo K, Arai F, et al. Regulation of reactive Oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J Immunol 2007;178:103-10. [PubMed]
- Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 2007;128:325-39. [PubMed]
- Abbas HA, Maccio DR, Coskun S, et al. Mdm2 is required for survival of hematopoietic stem cells/progenitors via dampening of ROS-induced p53 activity. Cell Stem Cell 2010;7:606-17. [PubMed]
- Chen C, Liu Y, Liu R, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive Oxygen species. J Exp Med 2008;205:2397-408. [PubMed]
- Liu J, Cao L, Chen J, et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 2009;459:387-92. [PubMed]
- Miyamoto K, Araki KY, Naka K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 2007;1:101-12. [PubMed]
- Yalcin S, Zhang X, Luciano JP, et al. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem 2008;283:25692-705. [PubMed]
- Rodriguez JM, Elias F, Montaner A, et al. Oligonucleotide IMT504 induces an immunogenic phenotype and apoptosis in chronic lymphocytic leukemia cells. Medicina 2006;66:9-16. [PubMed]
- Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 2004;431:997-1002. [PubMed]
- Schuringa JJ, Vellenga E. Role of the polycomb group gene BMI1 in normal and leukemic hematopoietic stem and progenitor cells. Curr Opin Hematol 2010;17:294-9. [PubMed]
- Du W, Adam Z, Rani R, et al. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid Redox Signal 2008;10:1909-21. [PubMed]
- Oakley EJ, Van Zant G. Age-related changes in niche cells influence hematopoietic stem cell function. Cell Stem Cell 2010;6:93-4. [PubMed]
- Waterstrat A, Van Zant G. Effects of aging on hematopoietic stem and progenitor cells. Curr Opin Immunol 2009;21:408-13. [PubMed]
- Xing Z, Ryan MA, Daria D, et al. Increased hematopoietic stem cell mobilization in aged mice. Blood 2006;108:2190-7. [PubMed]
- Geiger H, Rennebeck G, Van Zant G. Regulation of hematopoietic stem cell aging in vivo by a distinct genetic element. Proc Natl Acad Sci U S A 2005;102:5102-7. [PubMed]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 2005;120:483-95. [PubMed]
- Pazhanisamy SK, Li H, Wang Y, et al. NADPH oxidase inhibition attenuates total body irradiation-induced haematopoietic genomic instability. Mutagenesis 2011;26:431-5. [PubMed]
- Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets 2013;14:1347-56. [PubMed]
- Scharpfenecker M, Floot B, Russell NS, et al. The TGF-β co-receptor endoglin regulates macrophage infiltration and cytokine production in the irradiated mouse kidney. Radiother Oncol 2012;105:313-20. [PubMed]
- Cadenas E, Davies KJ. Mitochondrial free radical Generation, oxidative stress, and aging. Free Radic Biol Med 2000;29:222-30. [PubMed]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 2001;81:807-69. [PubMed]
- Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol 2009;86:237-50. [PubMed]
- Wang W, Chen JX, Liao R, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol 2002;22:3389-403. [PubMed]
- Deng Q, Liao R, Wu BL, et al. High intensity ras signaling induces premature senescence by activating p38 pathway in primary human fibroblasts. J Biol Chem 2004;279:1050-9. [PubMed]
- Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells 2003;8:131-44. [PubMed]
- Haq R, Brenton JD, Takahashi M, et al. Constitutive p38HOG mitogen-activated protein kinase activation induces permanent cell cycle arrest and senescence. Cancer Res 2002;62:5076-82. [PubMed]
- Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta 2008;1780:1325-36.
- Chiang E, Dang O, Anderson K, et al. Cutting edge: apoptosis-regulating signal kinase 1 is required for reactive Oxygen species-mediated activation of IFN regulatory factor 3 by lipopolysaccharide. J Immunol 2006;176:5720-4. [PubMed]
- Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and Cancer. Cancer Metastasis Rev 2008;27:253-61. [PubMed]
- Patterson KI, Brummer T, O'brien PM, et al. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J 2009;418:475-89. [PubMed]
- Ostman A, Frijhoff J, Sandin A, et al. Regulation of protein tyrosine phosphatases by reversible oxidation. J Biochem 2011;150:345-56. [PubMed]
- Navas TA, Mohindru M, Estes M, et al. Inhibition of overactivated p38 MAPK can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood 2006;108:4170-7. [PubMed]
- Zhou L, Opalinska J, Verma A. p38 MAP kinase regulates stem cell apoptosis in human hematopoietic failure. Cell Cycle 2007;6:534-7. [PubMed]
- Li D, Wang Y, Wu H, et al. Mitigation of ionizing radiation-induced bone marrow suppression by p38 inhibition and G-CSF administration. J Radiat Res (Tokyo) 2011;52:712-6. [PubMed]
- Wang Y, Liu L, Zhou D. Inhibition of p38 MAPK attenuates ionizing radiation-induced hematopoietic cell senescence and residual bone marrow injury. Radiat Res 2011;176:743-52. [PubMed]
- Li D, Wang Y, Wu H, et al. The effects of p38 MAPK inhibition combined with G-CSF administration on the hematoimmune system in mice with irradiation injury. PLoS One 2013;8:e62921 [PubMed]
- Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev 2003;13:77-83. [PubMed]
- Sharpless NE, Depinho RA. The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev 1999;9:22-30. [PubMed]
- Cheng T. Cell cycle inhibitors in normal and tumor stem cells. Oncogene 2004;23:7256-66. [PubMed]
- Narita M, Nũnez S, Heard E, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003;113:703-16. [PubMed]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 2005;120:513-22. [PubMed]
- Robles SJ, Adami GR. Agents that cause DNA double Strand breaks Lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene 1998;16:1113-23. [PubMed]
- te Poele RH, Okorokov AL, Jardine L, et al. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 2002;62:1876-83. [PubMed]
- Beauséjour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 2003;22:4212-22. [PubMed]
- Sherr CJ, Weber JD. The ARF/p53 pathway. Curr Opin Genet Dev 2000;10:94-9. [PubMed]
- Molofsky AV, Pardal R, Iwashita T, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 2003;425:962-7. [PubMed]
- Stepanova L, Sorrentino BP. A limited role for p16Ink4a and p19Arf in the loss of hematopoietic stem cells during proliferative stress. Blood 2005;106:827-32. [PubMed]
- Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 2006;443:421-6. [PubMed]
- Ditch S, Paull TT. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem Sci 2012;37:15-22. [PubMed]
- Shi X, Zhang Y, Zheng J, et al. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal 2012;16:1215-28. [PubMed]
- Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst) 2002;1:3-25. [PubMed]
- Piccoli C, D'aprile A, Ripoli M, et al. Bone-marrow derived hematopoietic stem/progenitor cells Express multiple isoforms of NADPH oxidase and produce constitutively reactive Oxygen species. Biochem Biophys Res Commun 2007;353:965-72. [PubMed]
- Piccoli C, Ria R, Scrima R, et al. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J Biol Chem 2005;280:26467-76. [PubMed]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007;87:245-313. [PubMed]
- Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 2007;43:332-47. [PubMed]
- Yamaguchi M, Kashiwakura I. Role of reactive oxygen species in the radiation response of human hematopoietic stem/progenitor cells. PLoS One 2013;8:e70503 [PubMed]
- Serrander L, Cartier L, Bedard K, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS Generation. Biochem J 2007;406:105-14. [PubMed]
- Michaeloudes C, Sukkar MB, Khorasani NM, et al. TGF-β regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2011;300:L295-304. [PubMed]
- Zhang L, Sheppard OR, Shah AM, et al. Positive regulation of the NADPH oxidase NOX4 promoter in vascular smooth muscle cells by E2F. Free Radic Biol Med 2008;45:679-85. [PubMed]
- Singleton PA, Pendyala S, Gorshkova IA, et al. Dynamin 2 and c-Abl are novel regulators of hyperoxia-mediated NADPH oxidase activation and reactive Oxygen species production in caveolin-enriched microdomains of the endothelium. J Biol Chem 2009;284:34964-75. [PubMed]
- Bondi CD, Manickam N, Lee DY, et al. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol 2010;21:93-102. [PubMed]
- Sturrock A, Cahill B, Norman K, et al. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive Oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2006;290:L661-73. [PubMed]
- Li H, Liu Q, Wang N, et al. Correlation of different NADPH oxidase homologues with late endothelial progenitor cell senescence induced by angiotensin II: effect of telmisartan. Intern Med 2011;50:1631-42. [PubMed]
- Gorin Y, Ricono JM, Kim NH, et al. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol 2003;285:F219-29. [PubMed]
- Liu J, Zhang FF, Li L, et al. ClC-3 deficiency prevents apoptosis induced by angiotensin II in endothelial progenitor cells via inhibition of NADPH oxidase. Apoptosis 2013;18:1262-73. [PubMed]
- Boerma M, Wang J, Sridharan V, et al. Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart. PLoS One 2013;8:e70479 [PubMed]
- Kalaitzidis D, Sykes SM, Wang Z, et al. mTOR complex 1 plays critical roles in hematopoiesis and pten-loss-evoked leukemogenesis. Cell Stem Cell 2012;11:429-39. [PubMed]
- Lee JY, Nakada D, Yilmaz OH, et al. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell 2010;7:593-605. [PubMed]
- Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 2006;441:475-82. [PubMed]
- Zhang J, Grindley JC, Yin T, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 2006;441:518-22. [PubMed]
- Gan B, Sahin E, Jiang S, et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A 2008;105:19384-9. [PubMed]
- Yalcin S, Marinkovic D, Mungamuri SK, et al. ROS-mediated amplification of AKT/mTOR signalling pathway leads to myeloproliferative syndrome in Foxo3(-/-) mice. EMBO J 2010;29:4118-31. [PubMed]
- Alexander A, Cai SL, Kim J, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A 2010;107:4153-8. [PubMed]
- Handayaningsih AE, Iguchi G, Fukuoka H, et al. Reactive Oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology 2011;152:912-21. [PubMed]
- Eid AA, Ford BM, Bhandary B, et al. Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes 2013;62:2935-47. [PubMed]
- Toschi A, Lee E, Gadir N, et al. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. J Biol Chem 2008;283:34495-9. [PubMed]
- Finlay DK, Rosenzweig E, Sinclair LV, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med 2012;209:2441-53. [PubMed]
- Diebold I, Petry A, Hess J, et al. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 2010;21:2087-96. [PubMed]
- Bonello S, Zähringer C, Belaiba RS, et al. Reactive Oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol 2007;27:755-61. [PubMed]
- Marusyk A, Porter CC, Zaberezhnyy V, et al. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol 2010;8:e1000324 [PubMed]


