
Review on liver transplant for hepatocellular carcinoma
Introduction
The first solid organ transplant in the modern era was performed in 1883 by Dr. Theodor Kocher, who successfully implanted thyroid tissues in post-thyroidectomy patients (1). The concept of replacing a failed organ through transplant was widely acknowledged soon thereafter. In 1963, Dr. Thomas Starzl performed the first human liver transplant. OLT became the standard of care for end stage liver disease (ESLD) in the 1980s, especially with the invention of various immunosuppressants. Today, the success of OLT is marked by a 1- and 5-yr survival of 85% and 70% (2), in an otherwise terminal condition.
Hepatocellular carcinoma (HCC) is the most common primary liver cancer. In some parts of Southeast Asia, it is the most common malignancy, in part due to the endemic spread of Hepatitis B and C viruses. Other common risk factors for developing HCC include cirrhosis, alcohol, and non-alcoholic fatty liver disease. Overall, HCC has become more prevalent globally, causing 250,000 to 1 million deaths annually worldwide (3). Without treatment, HCC has a high mortality rate, with a 5-year survival of 10% (4). OLT offers a potential cure for HCC, especially if the cancer is found in early stages (T1 or T2). Unfortunately, the worldwide shortage of deceased liver donors presents a challenge to justifiably distribute liver grafts among patients in need of OLT.
Epidemiology and overview
In the years prior to 2002, the overall 5-year survival for HCC was merely 11.7% (5). However, it drastically improved over the last decade, due to earlier diagnosis from better cancer screening, and new treatment options, from locoregional therapy (LRT) to OLT.
Liver allocation has come a long way. In the 1980’s, distribution of this scarce resource used to be “ad hoc” basis, solely determined by medical providers. In the 1990’s, ICU patients and hospital patients had priority over clinic patients, considering that inpatients are likely to have a higher mortality without immediate intervention. In 1998, minimal listing criteria were instituted using the Child-Turcotte-Pugh (CTP) score (6). This scoring system takes into account encephalopathy, ascites, bilirubin, albumin, and pro-thrombin time. A numeric score was then converted to class A, B, or C, with C being on the more severe end of the spectrum. Despite its seemingly comprehensive determinants, this score became quite subjective, requiring physicians to accurately stage hepatic encephalopathy and ascites.
Finally, in 2002, the Model of End Stage Liver Disease (MELD) score was adopted in prioritizing patients for liver transplant (7). This score was initially developed to predict mortality in patients with complications of portal hypertension undergoing transjugular intrahepatic portosystemic shunt placement (TIPS) (8). It is calculated based on three objective variables: international normalized ratio (INR), bilirubin, and serum creatinine. This score was subsequently found to be also useful in predicting three months mortality in patients with liver disease, and thus is currently used to prioritize deceased donor liver allocation. Disadvantage of this scoring system is that it does not take into account quality of life issues, such as when hepatic encephalopathy or ascites can be detrimental to patients’ lives. Other than the MELD score along, there are several other factors that go into candidacy for a liver transplant, including BMI, social support, cardiac/pulmonary status, portal vein patency, and other malignancy or co-morbidities.
In regards to allocation, the United States is divided into 11 different regions. Deceased donor livers that become available in a certain region can be shared amongst those living within the region (9) (unos.org). The higher the MELD score, the higher on the list one becomes. However, every region has a different MELD average for receiving a liver, thus making certain regions more favorable in receiving a liver than others. Currently, the national average MELD score for liver transplant is 27 (2). However, the average MELD score in some areas varies from 26-33, depending on blood type. On average, for patients with MELD 21-30, the mean waiting time to OLT is 128 days. For MELD score 31-40, mean time is approximately 29 days. Average wait time can differ drastically by regions, which has resulted in inequity in organ allocation between different areas of the country.
Diagnosis of HCC
In recent years, as a result of better cancer screening, patients with HCC are diagnosed earlier (10). Diagnosis of cancer often requires pathology confirmation; however, HCC is an exception. AASLD published its most recent guideline which states that lesions greater than 1 cm, with triple phase CT or MRI showing arterial enhancement, followed by portal venous phase washout, can confirm the diagnosis of HCC, without a liver biopsy (11). New nodules greater than 1cm in cirrhotic liver showing typical pattern of HCC are nearly 100% specific with high positive predictive power (12-14). If initial imaging does not show typical pattern, then a second imaging modality should be pursued. Atypical imaging pattern on CT or MRI, such as iso- or hypo-vascular enhancing lesion during arterial phase without portal washout should undergo biopsy (Figure 1). Major complications associated with a biopsy include bleeding and needle tract seeding of tumor, which has been reported in multiple cases (15). A large retrospective study done by Wang et al. in China showed a 0.2% risk of implantation of metastases and 0.4% risk of hemorrhage (16).
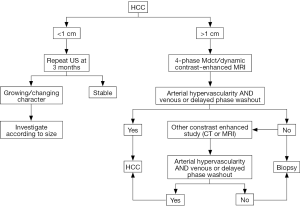
Molecular markers for detecting HCC
Elevations of alpha-fetoprotein level in the serum is not very sensitive (39-65%) nor specific (76-94%) for the diagnosis of HCC. Most recent AASLD guideline recommends against testing AFP to screen for HCC in cirrhotic patients. On the other hand, AFP has found a role in the monitoring of response and tumor progression after treatments. Diaz et al. observed that a reduction in serum AFP level after LRT predicts tumor reduction (17). Also, the pre-operative clinical prognostic factor for mortality and recurrence after treatment was AFP level higher than 300 ng/mL (18). In addition, AFP also has a role in predicting post-transplant outcomes. Several studies showed patients with significantly elevated AFP prior to transplant have poorer outcomes (19). Some experts even feel that AFP >1,000 ng/mL should be the criteria used to delist otherwise eligible patients. However, this is still an area of debate.
Staging of HCC
Once HCC is diagnosed, staging with either CT or MRI of the chest, abdomen and pelvis is required. The Barcelona Clinic Liver Cancer developed a staging system in 1999 that takes into account the performance status, characteristic of the tumor (single nodule or multi-nodular), vascular invasion, and presence of portal HTN. This BCLC classification system has become a widely accepted algorithm for all HCC patients in earlier disease, linking their current status prognosis with treatment recommendations. The widely accepted TNM staging system of many malignancy, although considered, seems to have inferior prognostic ability of long term survival for HCC, mostly because the severity of liver disease and complications of cirrhosis are not included as part of the staging system (20).
Indications of liver transplant listing: Milan criteria (MC)
When OLT initially became widely practiced, early work on transplanting patients with HCC had high post-OLT recurrence rate and subsequently high mortality. The poor outcome was in part related to the indiscrete selection of patients. Over the last two decades, investigators began to describe and define tumor characteristics that predict chance of recurrence after treatment and those associated with high mortality. In 1993, Bismuth et al. showed that those with at most three tumors, each less than 3 cm had a better outcome with OLT compared to surgical resection (21). In 1996, Mazzaferro et al. proposed the MC, which showed that patients with solitary HCC <5 cm or up to three lesions each smaller than 3 cm, without macrovascular invasion or extrahepatic spread, had a 5-year survival of 70% after OLT (22). This survival benefit is comparable to OLT in non-HCC population. Given the excellent outcome, MC has been adapted globally (EASL and AASLD guidelines) in selecting HCC patients for liver transplant (23,24). In addition, to acknowledge the high mortality of HCC (25), patients diagnosed with HCC are given priority listing in terms of extra points to match their mortality. Although MELD score is a useful tool to accurately predict high mortality in ESLD patients, it is less powerful for HCC patients (AASLD guideline). Therefore, to give HCC patients equal opportunity for OLT, they are given 22 points for solitary HCC 2-5 cm or three nodules each <3 cm. In addition, 10% point increase every three months due to estimated 15% mortality increase (26).
The adoption of the MC offered a promising 5-year post-OLT survival at 70%, in keeping with the non-HCC transplant group (22). Although Milan criteria is well validated (Table 1), the cutoff size and number are rather arbitrary. Thus, many find MC to be overly stringent, limiting a few potentially acceptable candidates from transplant. In addition, some argue that imaging may underestimate tumor size. Freeman et al. evaluated the UNOS database and reported that radiologic exams are not very precise, underestimating tumor load in 27% of the patients while overestimating in 30% of the population (30). Imaging technique, protocols, and expert interpretation are also variable among transplant centers. This further leads to questioning of the cutoff tumor number and size dictated by the MC. For these reasons, a number of experts are looking into expanding or modifying the criteria for OLT listing.
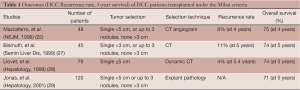
Full table
Expanding criteria
Although MC (one nodule <5 cm or up to three nodules, each <3 cm) outlines an acceptable risk to justifiably transplant HCC patients, the precise amount of tumor burden to be considered reasonable is not well established.
An attempt to expand beyond MC was done in 2001 by University California at San Francisco (UCSF). They developed the UCSF criteria: single nodule <6.5 cm; or multiple nodules with the largest <4.5 cm in diameter and the sum of total diameters <8 cm. Comparing UCSF to MC, the survival rate after transplant appeared to be similar (19). Although the results were exciting for those who do not initially qualify for MC, critics noted that in this study, only 24% of the population fell outside the MC. This may lead to dilution of poorer outcomes in those with larger tumors burden. Furthermore, the UCSF study is a retrospective analysis based on explants pathology, not pre-transplant radiology (31). The study included explant pathology and microvascular invasion (MVI) in the prognostic model, but these information are not usually available until post-OLT. A later paper by University California at Los Angeles (UCLA) with similar design validated the UCSF criteria, where 40% patients were outside MC but within UCSF (32). However, the UCSF criteria will need additional validation.
Another large meta-analysis was done by Mazzaferro et al. (33) in 2009 to study those individuals who do not fit into MC. The study included 1,556 patients transplanted from 36 centers. Their concept of expansion was termed “up to seven criteria”—number of tumors is up to seven, and the sum of tumor diameters up to 7 cm. The 5-year overall survival of this population after OLT is approximately 71.2%. They also initiated the “Metroticket concept”—the further one expands beyond MC, the more one pays in terms of higher recurrence and poorer post-OLT survival.
Currently, expansion beyond MC still requires more validation. Tumor recurrence may be under reported in OPTN database, thus no national data is available to support criteria for expansion (34). Many opponents of expansion criteria have shown that tumors exceeding the MC may have increased risk of MVI, microsatellites, and poorly differentiated tumor type (29,31,35-39)—all of which are associated with poorer outcomes. Therefore, the decision of expansion still falls on the individual centers to define the maximum cutoff size and number of HCC lesion at which the risk of recurrence may be considered acceptable. Another point of consideration is distributive justice. Due to the shortage in donor livers, this resource should be shared fairly among HCC and non-HCC patients. The post-OLT outcome of the expansion group must be similar or only slightly worse than the MC group to justify fair allocation. Volk et al. showed that a liberal approach to transplant selection would lead to a 44% increase in risk of death for all patients on the waitlist (40). He estimated that the 5-year post-OLT survival for HCC group needs to be at least 61%, to not have harmful effect on non-HCC group. To add to the complexity of this issue, there is regional variation in post-OLT success which muddies the nation-wide policy (34).
Downstaging to meet MC for transplant
The MC (single tumor <5 cm, or up to three tumors each <3 cm) is currently used for eligibility to OLT. For tumor burden beyond Milan, there are two ways to achieve a potential transplant. One is by expanding the criteria as explained above, and the other option is to undergo local regional therapy (LRT). LRT allows for shrinkage of tumor burden to meet MC, so one can be listed for OLT. This method is termed downstaging. There are several studies validating downstaging, and they are outlined in Table 2.

Full table
The technique of choice for downstaging is institution dependent. There is very limited head-to-head comparison between different procedures. While some studies follow RECIST (Response Evaluation Criteria in Solid Tumors) as down-staging measure, most use MC as an endpoint. Once the tumor burden is managed within an acceptable range, patients will be monitored closely for at least three months prior to listing (34,43,44). This process allows time to observe the behavior of the tumor. A period of waiting time prior to listing is not without benefit. It allows physician to select out those with aggressive tumors, therefore high risk for transplant. A study from Northwestern University, using living donor model, has hypothesized that fast track transplant for HCC has higher rate of recurrence post-OLT (45).
Currently, no clear guideline exists to exclude anyone from undergoing downstaging (41,46-48). However, distant metastasis or macrovascular invasion usually precludes patients from undergoing the procedure, given the high risk of recurrence. In the United States, only several regions have a clear down-staging protocol in place (43,47). Pomfret et al. proposed a limit on downstaging: single tumor <8 cm, or 2-3 tumors each <5 cm, with sum of tumor diameters <8 cm, exclude vascular invasion or number lesions >3. This proposal was raised in the 2010 report of national conference on liver allocation in patients with HCC. However, this proposal will need to be further validated (34).
LRT
Many techniques are available for LRT: transarterial chemoembolization (TACE), radiofrequency thermal ablation (RFA), radioembolization, resection, conformal-radiotherapy (CRT) and tyrosine kinase inhibitor sorafenib. The choice of technique is determined by location, size and number of lesions. It is also dependent on the expertise of the institution. The goal of LRT is two folds: one is to downstage the tumor, and the other is to help patient maintain on the transplant list during the waiting period if their tumor grew in size.
TACE utilizes intra-arterial injections of chemotherapeutic drug into the hepatic artery followed by Iodized oil (lipiodol) injection (49). It has been shown to decrease dropout rate (9-14%) (50,51), improve survival (52,53), and allows for longer wait time on the transplant list (211-274 days) (50,51). The term “drop-out” refers to delisting of patients due to tumor progression or complications of HCC that prohibits OLT. Moreover, some showed that TACE prior to transplant may even result in decreased post-transplant recurrence rate (17% vs. 36% non-treatment) (54-56). However, additional data and validation would be needed to prove that LRT in fact lower HCC recurrence or improve survival after transplant.
RFA employs electrical conduction and heat generated to ablate the HCC lesion. It is done under imaging guidance. This technique requires careful selection of patient to prevent tumor seeding (subcapsular tumors and direct nodule puncture) (57). One study done by Ng et al. showed complete tumor ablation in 92.7% of 192 patients. With a median follow-up of 26 months, local recurrence occurred in 28 patients (14.5%) (58).
Resection is rarely used in cirrhosis related HCC, but is the primary mode in non-cirrhotic HCC (59). CRT is an option for patients who failed other LRT or not eligible for other LRT due to the tumor anatomy (60). Sorafenib has been proven effective as well, but has high complication and is associated with high dropout rate (61).
LRT allows patients to stay on the list for a longer period of time and therefore decreasing overall dropout rate (33,62). Several studies showed a dropout rate of only 0-10% at 12 months for low grade tumor (T1 or T2 patients) treated with LRT (50,54,57,63). Another study reports dropout rate being as high as 30% without bridging therapy for those meeting MC. LRT is now widely accepted and practiced, with OPTN data showing that 65% of HCC patients received LRT prior to transplant (34). University of California in San Francisco (UCSF) conducted a review on patients undergoing LRT. They found that those who successfully underwent tumor reduction and subsequently transplant, the 5-year survival is approximately 84% (44). This high survival rate suggests LRT may benefit patients who initially do not meet MC.
Multi-phase CT or MRI should be performed 4-6 weeks after each LRT (34), to measure residual tumor burden. One can also monitor for serum level of AFP. Those with AFP <500 ng/mL have better response than those with AFP >1,000 ng/mL at initiation of down-staging (18,31). AFP in this scenario can be monitored for signs of recurrence in those patients whose AFP returns to normal after treatments.
Drop-outs and wait-list monitoring
Depending on different regions of the country, average wait time to liver transplant varies. However, the limited donor pool often leads to an inevitably long wait time. Longer wait time is associated with more drop-outs from the waitlist. For each month on the list without a liver transplant, the rate of drop-out is estimated to increase by 4.0% (28).
Major risk factors for tumor progression while on the waiting list include: length of wait time and tumor characteristics. UCSF reports a series of dropout rates for patients within MC: 0% dropout at 3 months, 11.0% at 6 months, 57.4% at 12 months, and 68.7% at 18 months (64). The total dropout rate for a median waiting time of 330 days was 22%. Large tumor size and multi-focality of the lesions also correlate to higher risk of tumor progression, thus higher dropout rates. Other factors associated with high dropout rate include resistance to LRT, and AFP >200 ng/mL. OPTN data shows that AFP <500 correlates with a 7.4% dropout, while AFP >1,000 is associated with 24.9% of dropout rate.
Tumor progression monitoring relies on imaging and serum biomarkers. The standard imaging used in most centers is contrast-enhanced CT or MRI (33). Although the interval to repeat imaging is unclear, AASLD recommends every 3-4 months after initial management. A technique under investigation is dual contrast MRI, which is thought to be more sensitive in detecting small HCC lesions than triple phase CT or MRI. Limited information is available on the use of AFP to follow patients on the waitlist. However, in patients whose serum AFP level was initially elevated, and returned to normal after treatment, a subsequent rise in AFP may suggest HCC recurrence.
If tumor progressed past MC on imaging, patient would be deactivated to undergo downstaging, or delisted for palliative treatment if distant metastasis or vascular invasion is found.
Post-transplant monitoring
Roayaie et al. reported that HCC patients post-OLT have 18.3% chance of eventual tumor recurrence (65). This group of patients received OLT from 1988-2002; and the median time to tumor recurrence was 12.3 months. Interestingly, the rate of tumor recurrence dropped from 25.5% down to 8-11% after the MC adoption. Tumor recurrence marked a poor prognosis, with median survival <12 months. The 5-year survival is 22% for the recurrent cohort comparing to 64% for its counterpart. Sites of recurrence include liver alone (16%), both intra and extra hepatic (31%), or extrahepatic alone (53%). Liver, lungs and bones are most frequent metastatic organs. The risk factors of tumor reappearance include tumor size, number of lesions, tumor differentiation, MVI and regional lymph node involvement (66). There is a positive correlation between the tumor burden prior to transplant and the cancer recurrence rate post-OLT. Similar to waitlist monitoring, post-OLT patients need routine imaging and biomarker surveillance. To detect HCC recurrence early, contrast CT, MR or PET/CT should be done every 6 months to yearly, for the first 3-5 years post OLT (67). Regular ultrasound and AFP are less accurate but also less expensive, may be applied every 3-6 months, up to five years post-OLT. Rise in AFP above 20 ng/mL in patients who had normal AFP should raise the suspicion of recurrence, and one should obtain imaging.
Treatment of HCC recurrence after orthotopic liver transplant (OLT)
Surgical resection is the best option for local HCC recurrence post-OLT. In one study, series of nine patients who underwent resection for HCC recurrence experienced survival rate similar to those who did not have recurrence (68). This surprising results, however, is subject to selection bias and small sample size. If patient is not eligible for resection due to size, location, or multiplicity, radiofrequency ablation or chemoembolization may be next best options. For extrahepatic recurrence post-OLT, surgical management requires those with good functional status, single lesion, and long interval from transplant to recurrence. Bone metastasis survival is especially poor, and most aim to palliate pain with external beam radiation and zoledronate (69).
The SHARP trial published in 2008 showed some benefit with sorafenib to treat unresectable advanced stage HCC (70). Teng et al. from Taiwan recently reported sorafenib improves overall survival in HCC post-OLT patients as well (71). The studied patients had pre-transplant tumor beyond MC. Using sorafenib as an adjuvant therapy, there was no tumor recurrence at two years in five studied patients. Using it as palliative therapy after recurrence, there is a trend for survival benefit (50% vs. 20% at 18 months) although not statistically significant (P=0.17).
Conclusions
The selection of HCC patients for liver transplant is not a trivial task. It requires a balance between maximizing benefit in HCC patients and minimizing harm to non-HCC patients due to the scarce resource. After this review, there is an obvious need to further validate the criteria that is currently being used. In addition, future research is required to unifying a set of guidelines in LRT and downstaging protocol.
Acknowledgments
Funding: None. No federal or industry support was received for this review article. Authors include Hellen Chiao, Edward Yang, and Catherine Frenette.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Liver Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.10.04). The series “Liver Cancer” was commissioned by the editorial office without any funding or sponsorship. CTF served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schlich T. The Origins of Organ Transplantation: Surgery and Laboratory Science, 1880-1930. University of Rochester Press, New York, 2010.
- Organ Procurement and Transplantation Network. OPTN/SRTR 2011 Annual Data Report. Liver. Available online: http://srtr.transplant.hrsa.gov/annual_reports/2011 (accessed Sept 10, 2013).
- Okuda K. Epidemiology of primary liver cancer. In: Primary Liver Cancer in Japan, Tobe T. eds. Springer-Verlag, Tokyo, 1992:3.
- Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62-7. [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2006. CA Cancer J Clin 2006;56:106-30. [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [PubMed]
- Wiesner RH, McDiarmid SV, Kamath PS, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl 2001;7:567-80. [PubMed]
- Kamath PS, Kim W. The model for end‐stage liver disease (MELD). Hepatology 2007;45:797-805. [PubMed]
- United Network for Organ Sharing. Questions and Answers for Transplant Candidates about Liver Allocation Policy. Available online: WWW.UNOS.org (accessed Sept 10, 2013).
- Sherman M. Epidemiology of hepatocellular carcinoma. Oncology 2010;78:7-10. [PubMed]
- Bruix J, Sherman MAmerican Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [PubMed]
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97-104. [PubMed]
- Luca A, Caruso S, Milazzo M, et al. Multidetector-row computed tomography (MDCT) for the diagnosis of hepatocellular carcinoma in cirrhotic candidates for liver transplantation: prevalence of radiological vascular patterns and histological correlation with liver explants. Eur Radiol 2010;20:898-907. [PubMed]
- Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2010;59:638-44. [PubMed]
- Takamori R, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: Is needle biopsy of the liver always necessary? Liver Transpl 2000;6:67-72. [PubMed]
- Wang P, Meng ZQ, Chen Z, et al. Diagnostic value and complications of fine needle aspiration for primary liver cancer and its influence on the treatment outcome-a study based on 3011 patients in China. Eur J Surg Oncol 2008;34:541-6. [PubMed]
- Riaz A, Ryu RK, Kulik LM, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol 2009;27:5734-42. [PubMed]
- Figueras J, Ibanez L, Ramos E, et al. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: results of a multicenter study. Liver Transpl 2001;7:877-83. [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [PubMed]
- Lei HJ, Chau GY, Lui WY, et al. Prognostic value and clinical relevance of the 6th Edition 2002 American Joint Committee on Cancer staging system in patients with resectable hepatocellular carcinoma. J Am Coll Surg 2006;203:426-35.
- Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg 1993;218:145-51. [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [PubMed]
- Zavaglia C, De Carlis L, Alberti AB, et al. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol 2005;100:2708-16. [PubMed]
- Löhe F, Angele MK, Gerbes AL, et al. Tumour size is an important predictor for the outcome after liver transplantation for hepatocellular carcinoma. Eur J Surg Oncol 2005;31:994-9. [PubMed]
- Washburn K, Edwards E, Harper A, et al. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant 2010;10:1643-8. [PubMed]
- Freeman RB, Wiesner RH, Edwards E, et al. Results of the first year of the new liver allocation plan. Liver Transpl 2004;10:7-15. [PubMed]
- Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis 1999;19:311-22. [PubMed]
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434-40. [PubMed]
- Jonas S, Bechstein WO, Steinmüller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001;33:1080-6. [PubMed]
- Freeman RB, Mithoefer A, Ruthazer R, et al. Optimizing staging for hepatocellular carcinoma before liver transplantation: A retrospective analysis of the UNOS/OPTN database. Liver Transpl 2006;12:1504-11. [PubMed]
- Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 2007;7:2587-96. [PubMed]
- Duffy JP, Vardanian A, Benjamin E, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg 2007;246:502-9. [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [PubMed]
- Pomfret EA, Washburn K, Wald C, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl 2010;16:262-78. [PubMed]
- Khakhar A, Solano E, Stell D, et al. Survival after liver transplantation for hepatocellular carcinoma. Transplant Proc 2003;35:2438-41. [PubMed]
- Cillo U, Vitale A, Grigoletto F, et al. Intention-to-treat analysis of liver transplantation in selected, aggressively treated HCC patients exceeding the Milan criteria. Am J Transplant 2007;7:972-81. [PubMed]
- Herrero JI, Sangro B, Pardo F, et al. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl 2008;14:272-8. [PubMed]
- Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant 2008;8:2547-57. [PubMed]
- Marshall AE, Rushbrook SM, Vowler SL, et al. Tumor recurrence following liver transplantation for hepatocellular carcinoma: role of tumor proliferation status. Liver Transpl 2010;16:279-88. [PubMed]
- Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant 2008;8:839-46. [PubMed]
- Roayaie S, Frischer JS, Emre SH, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg 2002;235:533-9. [PubMed]
- Silva M, Moya A, Berenguer M, et al. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl 2008;14:1449-60. [PubMed]
- Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008;48:819-27. [PubMed]
- Fix OK, Kerlan R, Hirose R, et al. Five-year intention-to-treat outcome of down-staging of hepatocellular carcinoma prior to liver transplantation Hepatology 2009;50:305A. [abstract].
- Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology 2004;127:S277-82. [PubMed]
- Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg 1997;226:688-701. [PubMed]
- Yao FY, Hirose R, LaBerge JM, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl 2005;11:1505-14. [PubMed]
- Chapman WC, Majella Doyle MB, Stuart JE, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg 2008;248:617-25. [PubMed]
- Harada T, Matsuo K, Inoue T, Tamesue S, Nakamura H. Is preoperative hepatic arterial chemoembolization safe and effective for hepatocellular carcinoma? Ann Surg 1996;224:4. [PubMed]
- Maddala YK, Stadheim L, Andrews JC, et al. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transpl 2004;10:449-55. [PubMed]
- Millonig G, Graziadei IW, Freund MC, et al. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 2007;13:272-9. [PubMed]
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [PubMed]
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71. [PubMed]
- Porrett PM, Peterman H, Rosen M, et al. Lack of benefit of pre-transplant locoregional hepatic therapy for hepatocellular cancer in the current MELD era. Liver Transpl 2006;12:665-73. [PubMed]
- Oldhafer KJ, Chavan A, Frühauf NR, et al. Arterial chemoembolization before liver transplantation in patients with hepatocellular carcinoma: marked tumor necrosis, but no survival benefit? J Hepatol 1998;29:953-9. [PubMed]
- Pérez Saborido B, Meneu JC, Moreno E, García I, Moreno A, Fundora Y. Is transarterial chemoembolization necessary before liver transplantation for hepatocellular carcinoma? Am J Surg 2005;190:383-7. [PubMed]
- Mazzaferro V, Battiston C, Perrone S, et al. Radiofrequency Ablation of Small Hepatocellular Carcinoma in Cirrhotic Patients Awaiting Liver Transplantation: a prospective study. Ann Surg 2004;240:900-9. [PubMed]
- Ng KK, Poon RT, Lo CM, et al. Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. J Gastrointest Surg 2008;12:183-91. [PubMed]
- Llovet JM, Mas X, Aponte JJ, et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut 2002;50:123-8. [PubMed]
- Sandroussi C, Dawson LA, Lee M, et al. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int 2010;23:299-306. [PubMed]
- Vitale A, Volk ML, Pastorelli D, et al. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: a cost-benefit analysis while awaiting data on sorafenib safety. Hepatology 2010;51:165-73. [PubMed]
- Bhoori S, Sposito C, Germini A, et al. The challenges of liver transplantation for hepatocellular carcinoma on cirrhosis. Transpl Int 2010;23:712-22. [PubMed]
- Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 2005;41:1130-7. [PubMed]
- Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl 2003;9:684-92. [PubMed]
- Roayaie S, Schwartz JD, Sung MW, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl 2004;10:534-40. [PubMed]
- Hollebecque A, Decaens T, Boleslawski E, et al. Natural history and therapeutic management of recurrent hepatocellular carcinoma after liver transplantation. Gastroenterol Clin Biol 2009;33:361-9. [PubMed]
- Roberts JP. Tumor surveillance-what can and should be done? Screening for recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl 2005;11:S45-6. [PubMed]
- Taketomi A, Fukuhara T, Morita K, et al. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol 2010;17:2283-9. [PubMed]
- Montella L, Addeo R, Palmieri G, et al. Zoledronic acid in the treatment of bone metastases by hepatocellular carcinoma: a case series. Cancer Chemother Pharmacol 2010;65:1137-43. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Teng CL, Hwang WL, Chen YJ, et al. Sorafenib for hepatocellular carcinoma patients beyond Milan criteria after orthotopic liver transplantation: a case control study. World J Surg Oncol 2012;10:41. [PubMed]


