
The expression and prognostic value of hypoxia-inducible factor-1α and p53 in non-small cell lung carcinoma
Introduction
Lung cancer is the most common malignancy and leading cause of cancer-related death worldwide (1). Non-small cell lung carcinoma (NSCLC) accounts for 75–85% of all lung cancer cases. Despite great advances in diagnosis and therapy, the 5-year survival rate of lung cancer patients remains poor and was reported to be approximately 17% (2). Tumor relapse and metastasis are the main causes of mortality. Several molecular markers have been demonstrated in the literature that are closely related to tumor oncogenesis and progression. Therefore, the detection and analysis of the molecular markers may provide predictive information for the prognosis and may help to optimize treatment strategies.
Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor that mainly responds to hypoxic stress through two transactivation domains: the NH2-terminal and the COOH-terminal domains (3). It determines the activity of the HIF system and belongs to the basic helix-loop-helix-PAS protein family (4). Activated HIF-1α can help tumor cells adapt to a low oxygen environment by regulating downstream genes that play a crucial role in the basic biological processes of tumor survival and progression. HIF-1α protein has a very short half-life (approximately 5 min) and declines rapidly under normoxia (3). The expression of HIF-1α can be stabilized and accumulated under hypoxia, which occurs in most solid tumors. Under some normoxic conditions, HIF-1α can also be transcribed and synthesized through various signaling factors involving growth factors, cytokines and other signaling molecules (5-7).
HIF-1α expression was reported to be associated with tumor angiogenesis, metastasis, poor prognosis and resistance to therapy in several carcinoma cells (8,9). Neoplasm proliferation and angiogenesis are promoted by elevated HIF-1α expression, whereas the metastasis of cancer cells and angiogenesis are prohibited by down-regulating HIF-1α expression. Overexpression of HIF-1α in colorectal cancer (10), liver cancer (11), and pancreatic cancer (12) patients is more likely to be associated with unfavorable survival. However, the prognostic significance of HIF-1α in NSCLC is inconsistent (13,14). The radiation resistance is partly derived from the presence of hypoxia, and hypoxia-mediated radioresistance was reported to be dependent on the HIF-1α pathway, but the correlation between HIF-1α and radioresistance was only explored in preclinical studies (15-20).
The p53 tumor suppressor gene is the most commonly identified genetic alteration in human carcinoma. The expression of HIF-1α is down-regulated through binding to wild-type p53 protein (21). Aberration of the p53 gene is closely associated with oncogenesis. In normal tissue, the p53 gene plays an important role in maintaining genomic stability by promoting DNA repair, arresting aberrant cells and inducing apoptosis during DNA damage (22). Wild-type p53 protein is a negative regulator of cell proliferation and a positive promoter of apoptosis and often undergoes quick degradation. Mutation of the p53 gene may prolong the half-life of p53 protein, which usually results in the structural stabilization and accumulation of p53 protein that can be detected by immunohistochemistry (IHC) (23). The inactivation of p53’s normal function may be an inducer of increasing malignancy and resistance to chemotherapy (24). The prognostic value of p53 protein overexpression is unclear. Therefore, an intensive understanding of the prognosis of p53 and HIF-1α in NSCLC may result in a more reasonably targeted therapeutic regimen.
The aim of this study was to explore the overexpression of p53 protein and HIF-1α and their prognostic value in NSCLC patients. Additionally, the relationship between HIF-1α and p53 overexpression and resistance to therapy was also analyzed. The correlation between HIF-1α and epidermal growth factor receptor (EGFR) expression is discussed as well.
Methods
Patients and tissue characteristics
Fifty paraffin-embedded NSCLC tumor samples and seven benign lung lesion specimens between September 2001 and February 2004 were randomly selected in this study. Informed consent from all patients and approval from the Medical Ethics Committee of West China Hospital, Sichuan University, were obtained. All patients had received pulmonary lobectomy and mediastinum lymphadenectomy with R0 surgery and did not take any preoperative therapy. There were 32 males and 18 females, aged 43–76 years (mean age: 56 years). All the patient samples were histologically subtyped and graded according to the 3rd WHO (World Health Organization) classification (25) for lung cancer. Among them, there were 21 squamous cell carcinomas, 26 adenocarcinomas, and 3 adenosquamous carcinomas; additionally, 12 were poorly differentiated (G3), and 38 were moderate or well differentiated (G1–G2). Sixteen patients were staged I–II and 34 were staged III-IV based on the 7th edition AJCC (American Joint Committee of Cancer) staging system (26) for lung cancer. Thirty-five cases had intra-thoracic nodal metastasis (N1–3), whereas 15 patients were without lymph node metastasis (N0). All patients underwent 4 cycles of adjuvant platinum-based doublet chemotherapy, and 23 patients staged IIIA with N2 received postoperative elective mediastinal radiotherapy. The benign lung lesions were proven to be inflammatory tissue and were used as controls. Overall survival was calculated from the date of surgical resection to death.
Immunohistochemistry (IHC)
IHC was performed strictly according to the manufacturer’s instructions. The paraffin-embedded tumor samples were cut consecutively into 4-µm-thick sections and were deparaffinized and rehydrated in xylene and ethanol, respectively. Antigen retrieval was accomplished by microwaving the slices treated with sodium citrate buffer. After the slides were washed in distilled water, the tumor specimens were covered with 3% H2O2 for 5 minutes to exclude endogenous peroxidase. Non-specific antigen was blocked with normal goat serum for 10 minutes. The slides were exposed to the primary mouse monoclonal anti-EGFR antibody (Chemicon International, Inc.) diluted 1:200 for 30 minutes. The tissue sections were also incubated at 4 °C overnight with mouse monoclonal antibody against HIF-1α and p53 (Beijing Zhongshan Biological Company) at a dilution of 1:100. The slides were incubated with a biotinylated secondary anti-mouse antibody for 10 minutes and streptavidin-biotin peroxidase complex solution for 20 minutes. The slices were visualized with diaminobenzidine-tetrahydrochloride (DAB) substrate chromogen solution and then were counterstained with hematoxylin.
Scoring method
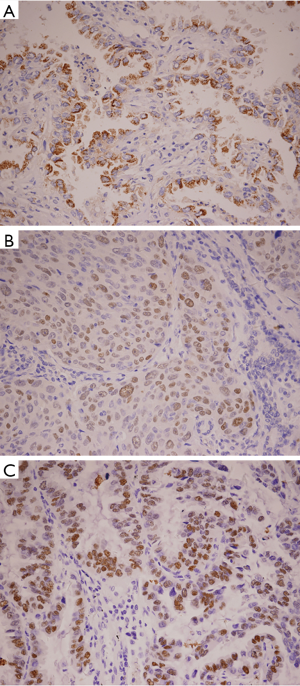
HIF-1α-positive cells were identified as exhibiting brown-stained nuclei or brownish-yellow particles, p53-positive cells showed brown granules in the nucleus, and EGFR-positive cells showed brownish-yellow granules in the cytoplasm and cell membrane. Evaluation of the immunohistochemistry results was performed independently by two pathologists who were blinded to all characteristics. The number of stained cells was counted in each field of 200 tumor cells. The positivity ratio comes from the average of 5 random fields under high power (200×). In our immunohistochemistry study, more than 50% nuclear staining of HIF-1α or p53 protein was defined as positive overexpression, while ≥10% EGFR staining was considered positive overexpression.
Statistical analysis
The data were analyzed by SPSS 24.0 version. The chi-squared test was used to examine the association of HIF-1α and p53 overexpression with various clinicopathologic parameters, as well as the relationship between HIF-1α and EGFR. The overall survival of NSCLC patients with HIF-1α or p53 protein overexpression was evaluated using the Kaplan–Meier method and log-rank test. For multivariate analysis of the prognostic factors, the Cox proportional-hazard model was used. P<0.05 was considered statistically significant.
Results
Overexpression of HIF-1α and p53 proteins
The NSCLC tumor showed remarkably different overexpression of HIF-1α and p53 protein from non-neoplastic lung tissue (P=0.045 and P=0.039, respectively) (Figure 1). Among them, 40% of tumor samples showed HIF-1α overexpression and 42% of cases displayed p53 protein overexpression (Tables 1,2). The positive rate of HIF-1α was 53.85% for adenocarcinoma, which was significantly higher than that of 23.81% for squamous carcinoma (P=0.037). The positive rate of p53 protein in adenocarcinoma was 34.60%, which was lower than that in squamous carcinoma (47.60%), but with no significant difference between them (P=0.382).

Full table

Full table
Correlation between HIF-1α or p53 protein overexpression and overall survival
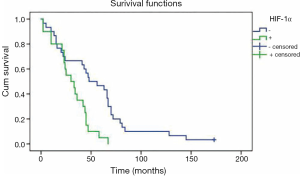
Patients with negative HIF-1α overexpression had a mean overall survival time of 55 months, which was significantly longer than that of 31 months for HIF-1α overexpression (P=0.002) (Figure 2). Cox multivariate analysis of survival showed that HIF-1α overexpression alone was not an independent prognostic factor. Meanwhile, no correlation was found between p53 protein overexpression and overall survival for NSCLC patients in univariate analysis (P=0.131).
Correlation between HIF-1α or p53 overexpression and clinicopathological features
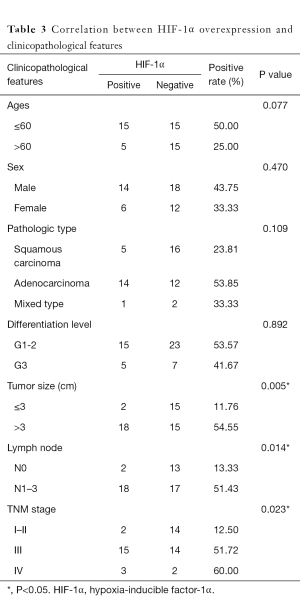
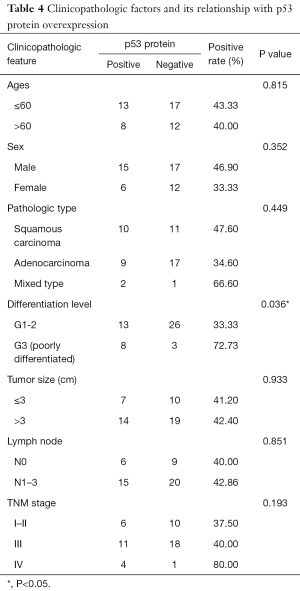
The overexpression of HIF-1α in different subgroups was compared and is summarized in Table 3, which shows that the difference in HIF-1α overexpression existed according to tumor size, nodal metastasis and TNM stage subgroup. The HIF-1α overexpression rate in patients with a smaller tumor size (≤3 cm) was significantly lower than that in patients with a larger tumor size (>3 cm) (11.76% vs. 54.55%, respectively; P=0.005). HIF-1α overexpression in lymph node metastasis cases was significantly higher than in cases without metastasis (51.43% vs. 13.33%, respectively; P=0.014). In addition, with higher TNM stage, the patients were more likely to present with HIF-1α overexpression, and the rates were 12.50%, 51.72% and 60% for those with stage I–II, III and IV disease, respectively (P=0.023). There was no significant difference in HIF-1α overexpression among other clinicopathological features (such as age, gender and differentiation level). By contrast, only the histological differentiation level showed statistical significance with p53 protein overexpression (P=0.036), with a significantly higher overexpression rate in poorly differentiated tumors. Although p53 protein overexpression was found to be higher in patients aged ≤60 years, male patients, and those with squamous carcinoma histology, poorly differentiated tumors, lymph node metastasis and stage III–IV disease, no statistical significance was detected (P>0.05) (Table 4).
Full table
Full table
HIF-1α overexpression in patients receiving radiotherapy
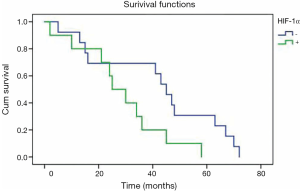
Twenty-three patients staged as IIIA with N2 had received postoperative adjuvant radiotherapy of the mediastinal lymph nodal region, and the mean survival time for patients with positive HIF-1α overexpression (n=10) was 29 months, which was significantly shorter than that (42 months) for patients (n=13) with negative HIF-1α overexpression (P=0.047) (Figure 3).
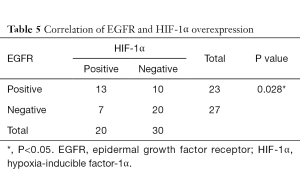
Correlation of HIF-1α with p53 overexpression and EGFR status
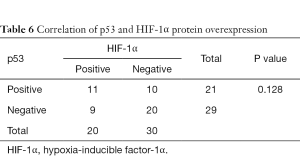
As shown in Tables 5,6, there was a significant correlation between EGFR and HIF-1α overexpression (P=0.028). However, no correlation was found between HIF-1α and p53 protein overexpression (P=0.128).
Full table
Full table
Discussion
HIF-1α is a predominant transcription factor that regulates the transcriptional activity of genes responding to hypoxic stress. Hydroxylation of HIF-1α proline residues leads to the degradation of HIF-1α protein under normoxic conditions. The activation of HIF-1α can be modulated by several proteins such as pVHL, CBP/P300 and p53 (21). Both HIF-1α and p53 abnormality make contributions to the tumorigenesis of lung epithelial cells. p53 alteration can be detected by IHC and gene sequencing. IHC was adopted in our study because of its reproducibility and high accordance with the genomic state (27,28). It is unclear whether the overexpression of HIF-1α and p53 protein has an adverse influence on the overall survival of NSCLC patients. Our study explored the prognostic value of HIF-1α and p53 overexpression in NSCLC patients by immunohistochemical testing.
HIF-1α and NSCLC
It was previously published that HIF-1α is overexpressed in human carcinomas (29). In our study, the positive rate of HIF-1α overexpression was 40% in NSCLC patients and was significantly higher than that in benign lung lesion tissue (40% vs. 0%, respectively; P=0.045), indicating that HIF-1α overexpression might have a strong association with carcinogenesis, tumor progression and metastasis in NSCLC. Previous IHC results showed 32–56% positivity rates of HIF-1α protein overexpression in NSCLC (30-32).
In our study, HIF-1α overexpression in NSCLC was significantly correlated with tumor size (P=0.005), lymph node metastasis (P=0.014) and TNM stage (P=0.023). In the tumor size subgroup analysis, HIF-1α overexpression was mainly distributed in patients with larger tumors. This finding could be explained by the fact that rapid tumor growth aggravated the hypoxic condition while molecular oxygen can diffuse a limited distance. HIF-1α overexpression is more likely to occur in patients with nodal metastasis and tumors of a greater size and higher stage, indicating a close link between HIF-1α and tumor genesis, progression and metastasis. HIF-1α overexpression was independent of the patient’s age, sex, and cell differentiation (P>0.05). Similar results were found in several studies (14). Prior studies showed that the positive rate in squamous cell carcinoma is higher than that in adenocarcinoma, but our study showed an opposite result (14). The small size of our study may be one possible interpretation for this discrepancy.
In our study, the Kaplan-Meier and log-rank analysis showed that patients with HIF-1α overexpression had a shorter mean overall survival time of 31 months compared with 55 months for patients without HIF-1α overexpression (P=0.002). Cox multivariate analysis adjusted by other significant variables demonstrated that HIF-1α was not an independent prognostic factor in NSCLC. The result was consistent with the finding from nasopharyngeal carcinoma that HIF-1α is only a risk factor for survival (33). Yang et al. recently reported a meta-analysis based on 20 studies published prior to January 2015 indicating that 25% of studies with elevated HIF-1α expression were associated with a poor prognosis in NSCLC patients (14). Therefore, HIF-1α might be a biomarker assisting in the identification of high risk for NSCLC patients. However, an opposite result was reported in a few studies wherein patients with HIF-1α positive expression had a longer survival than those with HIF-1α negative expression (9). Larger prospective trials are needed to ascertain the prognostic value of HIF-1α.
In patients receiving radiotherapy, the mean survival time for patients with HIF-1α overexpression (n=10) was 29 months, which was significantly shorter than the 42 months in patients (n=13) without HIF-1α overexpression (P=0.047). Thus, HIF-1α overexpression might be a molecular marker that is relevant to radioresistance. Hypoxia is a major influential factor of radioresistance. Up-regulation of HIF-1α often occurs in intratumoral hypoxia. Prior studies have demonstrated that HIF-1α could serve as a potential target to overcome hypoxia-relevant radioresistance. Small-molecule inhibitors or RNA interference (siRNA) targeting HIF-1α could sensitize carcinoma cells in irradiation for many tumor cell lines through cell cycle regulation and the apoptosis-related signaling pathway (15-20). However, the mechanism regarding how HIF-1α influences tumor radiation sensibility is complicated, and HIF-1α can induce radiation resistance via increasing the transcription of the apoptotic suppressor gene and promoting endothelial cell survival, while HIF-1α can also improve radiation sensitivity by ATP metabolism and proliferation (34). Rigorous clinical research is needed to explore the relationship between HIF-1α and radiation resistance.
Our study reported a significant correlation between EGFR and HIF-1α overexpression (P=0.028). Prior research showed that upregulation of HIF-1α can be mediated by EGFR activation through the PI3K/Akt/mTOR and Mek/Erk pathways (35,36). Park et al. reported that the prognostic value of HIF-1α was validated in EGFR negative expression (37), implying that the joint evaluation of biomarkers is more important in the prognosis.
p53 and NSCLC
In our study, the positive rate of p53 overexpression in NSCLC was 42%, a value that is apparently higher than that in benign lesion tissue (42% vs. 0%, respectively; P=0.039). Thus, p53 alteration might participate in the oncogenesis of NSCLC. Mitsudomi et al. showed that the positivity rate of p53 protein expression was correlated with the antibody used for detection, ranging from 17.5% to 76.8%, and the overall positive rate was 48.2% in NSCLC by IHC, which fits well with our study (23).
In our study, p53 protein overexpression in adenocarcinoma was lower than that in squamous carcinoma (34.6% vs. 47.6%, respectively), a result that agrees well with other study results (23,27,28,38). p53 protein overexpression was found to be higher in patients aged ≤60 years and those with squamous carcinoma, poorly differentiated tumors, a tumor size >3 cm, lymph node metastasis, and stage III–IV disease. However, among them, only the histological differentiation level showed statistical significance with p53 protein overexpression, with poorly differentiated tumors showing a significantly higher overexpression rate.
The present study showed no significant association between p53 protein overexpression and overall survival in NSCLC (P=0.131). A meta-analysis covering 22 studies showed that p53 alteration was a poor prognosis marker only in adenocarcinoma of NSCLC (23). In addition, similar results were found in several subsequent studies (39,40). In our study, no prognostic value of p53 overexpression was found in either squamous carcinoma or adenocarcinoma. However, the prognostic value of p53 expression in NSCLC has been inconsistent among different studies (23,27,28,39-41). The possible explanation might be that tumorigenesis and cancer development are complicated and influenced by various factors.
In 27 patients only receiving chemotherapy, we observed a mean overall survival of 47 months in patients (n=9) with p53 protein overexpression, which was shorter than 57 months for patients (n=18) without overexpression (P=0.443). Shiga et al. reported a similar result in head and neck cancer (42). In vitro studies have also identified drug resistance in p53-mutated lung cancer cells (24). Tung et al. proved the hypothesis that mutant p53 might induce cisplatin resistance via upregulating Nrf2 expression (43), implying that NSCLC patients with normal p53 function may benefit more from adjuvant chemotherapy.
In our study, no statistical correlation was found between p53 and HIF-1α protein overexpression. Both HIF-1α and tumor suppressor p53 mediate the cellular response to hypoxia stress. Loss of the p53 gene is correlated with upregulation of HIF-1α (21). p53 mutation could induce the resistance of p53-mediated apoptosis under hypoxia. In addition, HIF-1α could inhibit tumor cell apoptosis caused by irradiation via suppressing the expression of p53 (44). However, how the two factors interact to decide cell fate remains unclear.
In conclusion, our study illustrated that HIF-1α and p53 proteins are both overexpressed in NSCLC. HIF-1α might be a risk factor for overall survival and a biological marker relevant to radiation resistance. There was a significant correlation between HIF-1α and EGFR expression. Although our study failed to endorse the role of p53 abnormality as an unfavorable outcome in NSCLC, it clearly supports its critical role in the development of lung carcinoma. The prognostic value of HIF-1α and p53 expression needs further study.
Acknowledgments
Funding: This study was supported partly by the National Natural Science Foundation of China (No. 81573024).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent from all patients and the approval from Medical Ethics Committee of West China Hospital, Sichuan University has been obtained, and the ID is 2018(50).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin 2012;62:220-41. [Crossref] [PubMed]
- Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 2015;5:378-89. [Crossref] [PubMed]
- Ren W, Mi D, Yang K, et al. The expression of hypoxia-inducible factor-1α and its clinical significance in lung cancer: a systematic review and meta-analysis. Swiss Med Wkly 2013;143:w13855 [PubMed]
- Soni S, Padwad YS. HIF-1 in cancer therapy: two decade long story of a transcription factor. Acta Oncol 2017;56:503-15. [Crossref]
- Cheng JC, Klausen C, Leung PC. Hypoxia-inducible factor 1 alpha mediates epidermal growth factor-induced down-regulation of E-cadherin expression and cell invasion in human ovarian cancer cells. Cancer Lett 2013;329:197-206. [Crossref] [PubMed]
- Hiraki M, Kitajima Y, Kai K, et al. Knockdown of hypoxia-inducible factor-1alpha accelerates peritoneal dissemination via the upregulation of MMP-1 expression in gastric cancer cell lines. Exp Ther Med 2012;4:355-62. [Crossref] [PubMed]
- Minakata K, Takahashi F, Nara T, et al. Hypoxia induces gefitinib resistance in non-small-cell lung cancer with both mutant and wild-type epidermal growth factor receptors. Cancer Sci 2012;103:1946-54. [Crossref] [PubMed]
- Honguero Martínez AF, Arnau OA, Figueroa AS, et al. Prognostic value of the expression of vascular endothelial growth factor A and hypoxia-inducible factor 1alpha in patients undergoing surgery for non-small cell lung cancer. Med Clin (Barc) 2014;142:432-7. [PubMed]
- Baba Y, Nosho K, Shima K, et al. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol 2010;176:2292-301. [Crossref] [PubMed]
- Cao S, Yang S, Wu C, et al. Protein expression of hypoxia-inducible factor-1 alpha and hepatocellular carcinoma: a systematic review with meta-analysis. Clin Res Hepatol Gastroenterol 2014;38:598-603. [Crossref] [PubMed]
- Hoffmann AC, Mori R, Vallbohmer D, et al. High expression of HIF1a is a predictor of clinical outcome in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA, VEGF, and bFGF. Neoplasia 2008;10:674-9. [Crossref] [PubMed]
- Yan X, Jiao SC, Zhang GQ, et al. Tumor-associated immune factors are associated with recurrence and metastasis in non-small cell lung cancer. Cancer Gene Ther 2017;24:57-63. [Crossref] [PubMed]
- Yang SL, Ren QG, Wen L, et al. Clinicopathological and prognostic significance of hypoxia-inducible factor-1 alpha in lung cancer: a systematic review with meta-analysis. J Huazhong Univ Sci Technolog Med Sci 2016;36:321-7. [Crossref] [PubMed]
- Schwartz DL, Powis G, Thitai-Kumar A, et al. The selective hypoxia inducible factor-1 inhibitor PX-478 provides in vivo radiosensitization through tumor stromal effects. Mol Cancer Ther 2009;8:947-58. [Crossref] [PubMed]
- Moon SY, Chang HW, Roh JL, et al. Using YC-1 to overcome the radioresistance of hypoxic cancer cells. Oral Oncol 2009;45:915-9. [Crossref] [PubMed]
- Luo Z, Bai M, Xiao X, et al. Silencing of HIF-1α enhances the radiation sensitivity of human glioma growth in vitro and in vivo. Neuropharmacology 2015;89:168-74. [Crossref] [PubMed]
- Huang Y, Yu J, Yan C, et al. Effect of small interfering RNA targeting hypoxia-inducible factor-1α on radiosensitivity of PC3 cell line. Urology 2012;79:744.e17-24. [Crossref]
- Staab A, Fleischer M, Loeffler J, et al. Small interfering RNA targeting HIF-1α reduces hypoxia-dependent transcription and radiosensitizes hypoxic HT 1080 human fibrosarcoma cells in vitro. Strahlenther Onkol 2011;187:252-9. [Crossref] [PubMed]
- Kessler J, Hahnel A, Wichmann H, et al. HIF-1α inhibition by siRNA or chetomin in human malignant glioma cells. effect on hypoxic radioresistance and monitoring via CA49 expression. BMC Cancer 2010;10:605. [Crossref] [PubMed]
- Bae MK, Ahn MY, Jeong JW, et al. Jab1 interacts directly with HIF-1alpha and regulates its stability. J Biol Chem 2002;277:9-12. [Crossref] [PubMed]
- Kumari A, Bahl C, Singh N, et al. Association of p53 codon 72 polymorphism and survival of North Indian lung cancer patients treated with platinum-based chemotherapy. Mol Biol Rep 2016;43:1383-94. [Crossref] [PubMed]
- Mitsudomi T, Hamajima N, Ogawa M, et al. Prognostic Significance of p53 Alterations in Patients with Non-Small Cell Lung Cancer: A Meta-Analysis. Clin Cancer Res 2000;6:4055-63. [PubMed]
- Russo A, Saide A, Smaldone S, et al. Role of uL3 in Multidrug Resistance in p53-Mutated Lung Cancer Cells. Int J Mol Sci 2017;18. [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. World Health Organization Classification of Tumours: Pathology & Genetics Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press, 2004.
- Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual, 7th edition. New York, 2010.
- Smardova J, Liskova K, Hausnerova J, et al. Complex analysis of the p53 tumor suppressor in lung carcinoma. Oncol Rep 2016;35:1859-67. [Crossref] [PubMed]
- Mogi A, Kuwano H. TP53 mutations in nonsmall cell lung cancer. J Biomed Biotechnol 2011;2011:583929 [Crossref] [PubMed]
- Bottaro DP, Liotta LA. Cancer: out of air is not out of action. Nature 2003;423:593-5. [Crossref] [PubMed]
- Hung JJ, Yang MH, Hsu HS, et al. Prognostic significance of hypoxia-inducible factor1a, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax 2009;64:1082-9. [Crossref] [PubMed]
- Lu Y, Yu LQ, Zhu L, et al. Expression of HIF-1α and P-gp in non-small cell lung cancer and the relationship with HPV infection. Oncol Lett 2016;12:1455-1459. [Crossref] [PubMed]
- Ping W, Jiang WY, Chen WS, et al. Expression and Significance of Hypoxia Inducible Factor-1α and Lysyl Oxidase in Non-small Cell Lung Cancer. Asian Pac J Cancer Prev 2013;14:3613-8. [Crossref] [PubMed]
- Wan XB, Fan XJ, Huang PY, et al. Aurora-A activation, correlated with hypoxia-inducible factor-1α, promotes radiochemoresistance and predicts poor outcome for nasopharyngeal carcinoma. Cancer Sci 2012;103:1586-94. [Crossref] [PubMed]
- Zhang M, Qiu Q, Li Z, et al. HIF-1α regulates the response of primary sarcomas to radiation therapy through a cell autonomous mechanism. Radiat Res 2015;183:594-609. [Crossref] [PubMed]
- Lee JG, Wu R. Erlotinib-Cisplatin Combination Inhibits Growth and Angiogenesis through c-MYC and HIF-1α in EGFR-Mutated Lung Cancer In Vitro and In Vivo. Neoplasia 2015;17:190-200. [Crossref] [PubMed]
- Peng XH, Karna P, Cao Z, et al. Cross-talk between Epidermal Growth Factor Receptor and Hypoxia-inducible Factor-1 Signal Pathways Increases Resistance to Apoptosis by Up-regulating Survivin Gene Expression. J Biol Chem 2006;281:25903-14. [Crossref] [PubMed]
- Park S, Ha SY, Cho HY, et al. Prognostic implications of hypoxia-inducible factor-1alpha in epidermal growth factor receptor-negative non-small cell lung cancer. Lung Cancer 2011;72:100-7. [Crossref] [PubMed]
- Xu Y, Wang L, Zheng X, et al. Positive expression of p53, c-erbB2 and MRP proteins is correlated with survival rates of NSCLC patients. Mol Clin Oncol 2013;1:487-92. [Crossref] [PubMed]
- Huang CL, Yokomise H, Miyatake A. Clinical significance of the p53 pathway and associated gene therapy in non-small cell lung cancers. Future Oncol 2007;3:83-93. [Crossref] [PubMed]
- Nikliński J, Niklińska W, Laudanski J, et al. Prognostic molecular markers in non-small cell lung cancer. Lung Cancer 2001;34:S53-8. [Crossref] [PubMed]
- Xie D, Lan L, Huang K, et al. Association of p53/p21 expression and cigarette smoking with tumor progression and poor prognosis in non-small cell lung cancer patients. Oncol Rep 2014;32:2517-26. [Crossref] [PubMed]
- Shiga H, Health EI, Rasmussen AA, et al. Prognostic value of p53, glutathione S-transferase pi, and thymidylate synthase for neoadjuvant cisplatin-based chemotherapy in head and neck cancer. Clin Cancer Res 1999;5:4097-104. [PubMed]
- Tung MC, Lin PL, Wang YC, et al. Mutant p53 confers chemoresistance in non-small cell lung cancer by upregulating Nrf2. Oncotarget 2015;6:41692-705. [Crossref] [PubMed]
- Fu Z, Chen D, Cheng H, et al. Hypoxia-Inducible Factor-1a Protects Cervical Carcinoma Cells from Apoptosis Induced by Radiation via Modulation of Vascular Endothelial Growth Factor and p53 under Hypoxia. Med Sci Monit 2015;21:318-25. [Crossref] [PubMed]


