
This article has an erratum available at: http://dx.doi.org/10.21037/tcr.2019.04.17
Anti-GD2 CAR T cells could prove transformative for H3-K27M+ diffuse midline gliomas
Diffuse intrinsic pontine glioma (DIPG)-derived primary cultures and other diffuse midline glioma (DMG)-ones were served for screening of surface antigen expression and gene expression analysis of responsible glycosyltransferases. Disialoganglioside GD2 was widely and highly expressed in H3-K27M-mutant (H3-K27M+) glioma cells. Relevant glycosyltransferase genes underwent up-regulation as analyzed by quantitative reverse transcription polymerase chain reaction (RT-qPCR). As well known, gliomas are generally very malignant and resistant to any current therapies (1). Especially, DIPG are aggressive and universally fatal, and hard to even mitigate the symptoms, since it is difficult to dissect tumor tissues from surrounding normal ones because of its site of occurrence and invasive natures (2). Palliative radiation therapy has been performed, resulting in minimal improvement of the patients’ life. Chimeric antigen receptor (CAR)-expressing T cells have shown impressive effects on B cell malignancies. Thus, effects of anti-GD2 CAR T cells incorporating a 4-1BBz costimulatory domain were examined by adding to cultured tumor cells, and adoptive transfer into NSG mice bearing orthotopically-generated DMGs (3). Anti-GD2 CAR T cells demonstrated robust GD2-dependent cytokine release and killing of DMG cells in the in vitro system. For patient-derived H3-K27M+ DMG orthotopic xenografts, i.e., injection of anti-GD2 CAR T cells resulted in excellent effects to eliminate GD2-positive tumor cells, and to extend survival times of mice. Anti-GD2 CAR T cell administration was tolerated in the majority of mice bearing orthotopic xenografts, while peritumoral neuroinflammation during the acute phase of antitumor activity sometimes induced lethality of mice. Consequently, this novel approach should be very promising and could prove transformative for the lethal childhood cancers, provided that careful monitoring and aggressive neurointensive care management are prepared. Here, great significances of this study and some concerns to be solved before being applied in the clinical fields have been proposed.
Specificity of GD2 expression looks fine, but should be carefully examined
GD2 has never been demonstrated to be expressed in particular cell lineages or anatomical sites in the central nervous system (CNS). However, total brain extracts contain low but definite levels of GD2 (4). In peripheral nerves, expression of GD2 has been known through side effects of injection of anti-GD2 mAb for the treatment of malignant tumors such as neuroblastomas (5). Although GD2 has been known as a ganglioside recognized by host immune system, and to be expressed in neuroectoderm-derived tumors and normal brains at an early developmental stage (6), where GD2 is expressed in normal nerve tissues remains to be investigated.
Ganglioside GD2, structure and synthetic process
GD2 is an acidic glycosphingolipids (gangliosides) containing two sialic acids (disialyl ganglioside). GD2 is synthesized by β-1,4-N-acetylgalactosaminyl transferase (β-1,4-GalNAc-T) (B4GALNT1 product) from a precursor GD3. This enzyme can also catalyze the synthesis of GM2 from GM3, thereby being named as GM2/GD2 synthase (7). This cDNA clones were isolated by our group using an expression cloning system. Therefore, β-1,4-GalNAc-T is a key enzyme responsible for the synthesis of “complex gangliosides”. This was clearly demonstrated in the gene knockout of B4GALNT1, in which only GM3 and GD3 were remained (8). Thus, GD3 and GD2 are synthesized from GM3 via GD3 synthase (ST8SIA1) (9) or GD3 synthase and GM2/GD2 synthase, respectively (10). Therefore, GD3, GD2 and further extended species with two sialic acids on the inner galactose residue in ganglioside-core have been named b-series gangliosides as shown in Figure 1.
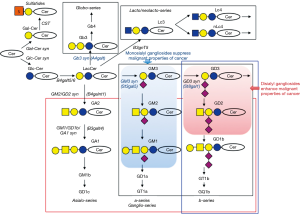
Whether ST8SIA1 is expressed in some cells or sites often becomes a key question to characterize general metabolic profiles of gangliosides in particular cells and tissues. In malignant tumors, ST8SIA1 is often expressed along with malignant transformation. Sometimes, expression of B4GALNT1 is expressed without or with ST8SIA1, leading to GM2 expression or GD3/GD2 expression as dominant gangliosides, respectively. By the way, β-1,4-GalNAc-T can also synthesize asialo-GM2 (GA2) from lactosylceramide (10). Although authors concluded that glycosyltransferase genes involved in the synthesis of glycolipids until GD2 were upregulated (3), combined expression of B4GALNT1 and ST8SIA1 seems to be critical for the specific expression of GD2. ST3GAL5 and B4GALT6 (and B4GALT5 also) are broadly expressed in many tissues and cells. Whether H3-K27 modification is directly involved in the up-regulation of B4GALNT1 (and ST8SIA1) seems of quite interest.
Systemic injection of anti-GD2 CAR T cells were well transferred to CNS and demonstrated great elimination of GD2 DMG xenografts
Since GD2 was identified as a melanoma-associated antigen recognized by host immune system (6) in the “autologous typing” study (11), GD2 has been a promising target of antibody therapy for neuroblastomas (5) and other tumors. Indeed, there have been a number of trials of immunotherapy for human cancers with various modification of anti-GD2 mAbs, i.e., combination of anti-GD2 mAb with other anti-cancer reagents (5,12), modification of structures in mAb molecules, and generation of GD2-mimic peptides (13). Among many clinical trials of anti-GD2 mAb, extension of the remission term in patients with neuroblastomas (14) seems to be the most striking result. Despite of big expect, anti-GD2 antibody therapy has not been well lead, and not obtained fully persuasive results yet.
Generally, target antigens for antibody therapy of tumors have been required to be function molecules expressed on the cell surface. Recent findings on GD2 as described above as well as older results (15,16) might indicate that GD2 is actually functional, and deserve to be a target of cancer immunotherapy.
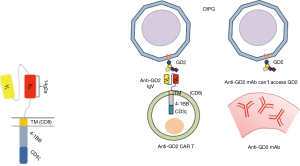
Supposed reasons for insufficient effects of anti-GD2 mAbs in these studies are as follows, (I) not enough killing activity of mAbs to eradicate all tumor cells, (II) not enough access of mAbs into tumor tissues, (III) heterogenous and/or inconsistent expression of GD2/GD3 on tumor cells. In Mount’s study, anti-GD2 CAR-T cells could infiltrate into glioma tissues in brain stem, and clearly eliminate tumor cells in meninges and tumor tissues (3). Transfer of T cells from peripheral blood vessels into CNS was markedly nice, and seemed to be much better than mAbs when systemically injected (Figure 2).
Functional aspects of GD2 in cancers and normal tissues
Contrastive effects of monosialyl and disialyl gangliosides in the regulation of malignant properties of tumor cells
Some of acidic glycosphingolipids, gangliosides have been considered as specific markers for neuroectoderm-derived tumors and leukemias. For example, GD3 was reported to be a melanoma-specific antigen (17), and GD2 was reported to be a neuroblastoma-associated antigen. GD2 was also revealed to be associated with small cell lung cancers (SCLCs), osteosarcomas, metastatic melanomas (18) and breast cancers (16). HTLV-I-infected T lymphocytes also expressed GD2 (19). These disialyl gangliosides have been shown to be involved in the malignant properties of cancer cells with some exception, whereas monosialyl gangliosides like GM3 and GM1 have been demonstrated to suppress malignant properties of cancer cells. Thus, gangliosides modulate cell phenotypes and signaling toward contrastive directions (Figure 1). Regulation of glycolipid-enriched microdomain (GEM)/rafts by gangliosides should be crucial, and have strong influence on the natures of GEM/rafts (20). GEM/rafts are microdomains on the cell membrane, consisting of high levels of cholesterol, sphingomyelin, GPI-anchored proteins as well as glycosphingolipids. Involvement of GEM/rafts in phenotypes of tumor cells has been increasingly recognized (20). In terms of tumor-promoting glycolipids, GD3 and GD2 have been well investigated.
Modes of action of disialyl gangliosides in cancer cells
In order to understand the regulatory functions of glycolipids in GEM/rafts, it seemed essential to identify glycolipid-recognizing ligand molecules. Among molecules that recognize glycolipids on the cell surface, cis-acting molecules with glycolipids seem to exert glycolipid functions. To identify interacting molecules with glycolipids in the vicinity of membrane, EMARS (enzyme-mediated activation of radical sources) reaction developed by Kotani et al. (21) has been used. Indeed, Neogenin was the representative molecule (22) that was localized in GEM/rafts only in GD3+ melanoma cells. PDGF receptor (PDGFR) was also identified as a GD3-interacting molecule in mouse glioma cells (23). It was shown that PDGFR, GD3 and Src family kinase Yes formed ternary complex, resulting in the dramatic enhancement of PDGF/PAGFR signals. As for GD2, ASCT-2 has been identified as a GD2-associating molecule in SCLC cells (24). ASCT-2 is a glutamine transporter, and expression of which resulted in the increased cell growth and invasion by enhancing the activation levels of downstream molecules of mTORC1 signaling axis such as p70 S6K1 and S6 (24).
Thus, both GD3 and GD2 play roles in the enhancement of malignant properties of tumor cells based on the collaboration with other membrane molecules. However, these two gangliosides show distinct expression patterns in the panel of tumor cells, and also in the profiles of individual associating molecules (unpublished results). In particular, in the case of tumors, in which GD2 is highly expressed and playing crucial roles in the malignant phenotypes, anti-GD2 CAR T should be extremely efficient as shown in this paper (3).
Disialyl gangliosides, GD3 and GD2 enhance malignant properties of human cancer cells
Since biochemical studies on melanoma gangliosides were reported (17), a number of murine or human mAbs for them have been reported (25). Then, genetic studies using glycosyltransferase cDNAs have been performed to investigate the roles of gangliosides in tumors. As for melanomas, p130Cas and paxillin were reported to undergo marked activation in GD3+ cells (26). Then, Src family kinase, Yes was reported to play as a crucial signaling molecule. These molecules were reported to form molecular complexes on the cell membrane. Furthermore, GD3 enhanced tumor cell adhesion to various extracellular matrices. Knockdown of integrin resulted in the suppression of phosphorylation levels of p130Cas, FAK and paxillin as well as adhesion intensity, suggesting that adhesion signals and growth factor/receptor signals seemed to cooperatively induce full activation of these molecules in GD3+ cells, suggesting that these pathways converge in GD3+ cells.
Among solid tumors, GD2 was characteristically expressed in SCLC, and the responsible glycosyltransferase genes were shown by us to be GM2/GD2 synthase and GD3 synthase (27). The former was expressed in all types of lung cancers, while the latter was expressed only in SCLC cells (27). Interestingly, anti-GD2 mAb induced apoptosis based on anoikis (28), suggesting that anti-GD2 mAbs can be utilized for the treatment of SCLC. Antibody therapy of SCLC targeting GD3 (29) or GD2 (30) is now starting.
As reported previously, majority of osteosarcoma cell lines expressed high levels of GD2. GD2/GD3 expression enhanced cell invasion and motility due to increased activation of either FAK or Lyn, leading to paxillin activation.
GD2 has been also detected in epithelial tumors like breast cancer. GD2 expression in breast cancer cells resulted in the increased cell proliferation and invasion (16). As an action mechanism of GD2, constitutive activation of HGF receptor, cMET in GD2+ cells was reported (16). Of interest, HGF/cMET signals were not mediated through lipid/rafts. This was also the case in melanoma cells. Roles of GD2 in melanomas remain to be examined, while its expression in the metastatic stage has been long well known (18).
Future scope and some concerns to be solved
Anti-GD2 CAR T cells could be expected to be translated for human cancers
As described above, roles of GD2 in cancer cells have been increasingly recognized. Furthermore, GD2 is recently considered to be a cancer stem cell marker for breast cancers (31). Its roles in glioma stem cells were also reported, although it could be a combination effects with GD3 (32). Recently, Iwasawa et al. also suggests that GD3/GD2 could be a nice candidate for immunotherapy for human gliomas (33), while their expression on human gliomas have been known. Not only DIPG in children, but adult gliomas can be objectives for anti-GD2 CAR T therapy, since antibody therapy by anti-PD-1 or anti-CTLA4 might have barriers in the drug access into tumor sites in CNS via blood brain barrier (BBB), even if those tumors express neoantigens (Figure 2).
GD2 (GD3) expression on functional T cells can be also a target of anti-GD2 CAR T cells
As authors described (3), careful monitoring and aggressive neurointensive care management will be required when anti-GD2 CAR T cells are applied for human gliomas. Moreover, specificity in the expression of GD2 needs to be carefully investigated toward whole human tissues and cells. It has been well known that GD3 is expressed on activated T cells and T cell lymphoblastic leukemia (ALL) cells (34), and GD2 is expressed on HTLV-1+ cells and adult T cell leukemia cell lines (19). Recently, it was reported that T cell receptor (TCR)-mediated signals induced GD2 expression in human peripheral T cells (35). Consequently, it is strongly suggested that highly immuno-competent T cells might express GD2 on the cell surface. Namely, anti-GD2 CAR-T might be also targets of killing by itself. This concern should be carefully clarified using human environments.
GvHD due to original TCR would be overcome
As the authors reported, the anti-GD2 CAR T cells may cause GvHD after relatively long days post-treatment (DPT) (~50) (3). This might be due to the TCR primarily expressed on anti-GD2 CAR-T cells. If so, suppression of original TCR gene expression by new genetic devices should be tried (36).
As emphasized in the article (3), I hope that anti-GD2 CAR T cell therapy for H3-K27M+ diffuse gliomas of pons, thalamus and spinal cord could prove transformative for these lethal childhood cancers, and also for fatal adult brain tumors.
Acknowledgments
We would thank Y Ohkawa, K Kaneko, N Esaki, N Hashimoto and K Hamamura for valuable discussion.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xian-Xin Qiu (Shanghai Proton and Heavy Ion Center (SPHIC), a.k.a. the Proton and Heavy Ion Center of Fudan University Shanghai Cancer Center (FUSCC), Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.08.21). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huse JT, Holland EC. Targeting brain cancer: advances in the moleculer pathology of malignant glioma and medulloblastoma. Nat Rev Cancer 2010;10:319-31. [Crossref] [PubMed]
- Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 2014;46:444-50. [Crossref] [PubMed]
- Mount CW, Majzner RG, Sundaresh S, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat Med 2018;24:572-9. [Crossref] [PubMed]
- Suzuki K. The pattern of mammalian brain gangliosides. II. Evaluation of the extraction procedures, postmortem changes and the effect of formalin preservation. J Neurochem 1965;12:629-38. [Crossref] [PubMed]
- Ahmed M, Cheung NK. Engineering anti-GD2 monoclonal antibodies for cancer immuneotherapy. FEBS Lett 2014;588:288-97. [Crossref] [PubMed]
- Watanabe T, Pukel CS, Takeyama H, et al. Human melanoma antigen AH is an autoantigenic ganglioside related to GD2. J Exp Med 1982;156:1884-9. [Crossref] [PubMed]
- Nagata Y, Yamashiro S, Yodoi J, et al. Expression cloning of β-1, 4 N-acetyl-galacto-saminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J Biol Chem 1992;267:12082-9. [PubMed]
- Takamiya K, Yamamoto A, Furukawa K, et al. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides, but exhibit only subtle defects in their nervous system. Proc Natl Acad Sci USA 1996;93:10662-7. [Crossref] [PubMed]
- Haraguchi M, Yamashiro S, Yamamoto A, et al. Isolation of GD3 synthase gene by expression cloning of GM3 alpha-2,8-sialyltransferase cDNA using anti-GD2 monoclonal antibody. Proc Natl Acad Sci USA 1994;91:10455-9. [Crossref] [PubMed]
- Yamashiro S, Haraguchi M, Furukawa K, et al. Substrate specificity of β-1,4-N-acetyl- galactosaminyltransferase in vitro and in cDNA-transfected cells. GM2/GD2 synthase efficiently generates asialo-GM2 in certain cells. J Biol Chem 1995;270:6149-55. [Crossref] [PubMed]
- Old LJ. Cancer immunology: the search for specificity--G. H. A. Clowes Memorial lecture. Cancer Res 1981;41:361-75. [PubMed]
- Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363:1324-34. [Crossref] [PubMed]
- Basak S, Birebent B, Purev E, et al. Induction of cellular immunity by anti-idiotypic antibodies mimicking GD2 ganglioside. Cancer Immunol Immunother 2003;52:145-54. [PubMed]
- Sait S, Modak S. Anti-GD2 immunotherapy for neuroblastoma. Expert Rev Anticancer Ther 2017;17:889-904. [Crossref] [PubMed]
- Furukawa K, Ohkawa Y, Yamauchi Y, et al. Fine tuning of cell signals by glycosylation. J Biochem 2012;151:573-8. [Crossref] [PubMed]
- Cazet A, Lefebvre J, Adriaenssens E, et al. GD3 synthase expression enhances proliferation and tumor growth of MDA-MB-231 breast cancer cells through c-Met activation. Mol Cancer Res 2010;8:1526-35. [Crossref] [PubMed]
- Portoukalian J, Zwingelstein G, Doré JF. Lipid composition of human malignant melanoma tumors at various levels of malignant growth. Eur J Biochem 1979;94:19-23. [Crossref] [PubMed]
- Thurin J, Thurin M, Herlyn M, et al. GD2 ganglioside biosynthesis is a distinct biochemical event in human melanoma tumor progression. FEBS Lett 1986;208:17-22. [Crossref] [PubMed]
- Furukawa K, Akagi T, Nagata Y, et al. GD2 ganglioside on human T-lymphotropic virus type I-infected T cells: Possible activation of β-1,4-N-acetylgalactosaminyl- transferase gene by p40tax. Proc Natl Acad Sci USA 1993;90:1972-6. [Crossref] [PubMed]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997;387:569-72. [Crossref] [PubMed]
- Kotani N, Gu J, Isaji T, et al. Biochemical visualization of cell surface molecular clustering in living cells. Proc Natl Acad Sci USA 2008;105:7405-9. [Crossref] [PubMed]
- Kaneko K, Ohkawa Y, Hashimoto N, et al. Neogenin, defined as a GD3-associated molecule by enzyme-mediated activation of radical sources, confers malignant properties via intracytoplasmic domain in melanoma cells. J Biol Chem 2016;291:16630-43. [Crossref] [PubMed]
- Ohkawa Y, Momota H, Kato A, et al. Ganglioside GD3 enhances invasiveness of gliomas by forming a complex with platelet-derived growth factor receptor α and Yes kinase. J Biol Chem 2015;290:16043-58. [Crossref] [PubMed]
- Esaki N, Ohkawa Y, Hashimoto N, et al. ASCT2 defined by enzyme-mediated activation of radical sources enhances malignancy of GD2-plus small cell lung cancer. Cancer Science 2018;109:141-53. [Crossref] [PubMed]
- Furukawa K, Lloyd KO. Gangliosides in melanoma. In: Ferrone S, editors. Human Melanoma: From Basic Research to Clinical Application. Heidelberg: Springer, 1993;15-30.
- Hamamura K, Furukawa K, Hayashi T, et al. Ganglioside GD3 promotes cell growth and invasion through p130Cas and paxillin in malignant melanoma cells. Proc Natl Acad Sci U S A 2005;102:11041-6. [Crossref] [PubMed]
- Yoshida S, Fukumoto S, Kawaguchi H, et al. Ganglioside G(D2) in small cell lung cancer cell lines: enhancement of cell proliferation and mediation of apoptosis. Cancer Res 2001;61:4244-52. [PubMed]
- Aixinjueluo W, Furukawa K, Zhang Q, et al. Mechanisms for the apoptosis of small cell lung cancer cells induced by anti-GD2 monoclonal antibodies: roles of anoikis. J Biol Chem 2005;280:29828-36. [Crossref] [PubMed]
- Blackhall FH, Shepherd FA. Small cell lung cancer and targeted therapies. Curr Opin Oncol 2007;19:103-8. [Crossref] [PubMed]
- Zhan J, Han Q, Wang K. Development of antibody therapeutics for small cell lung cancer. Expert Opin Investig Drugs 2013;22:235-44. [Crossref] [PubMed]
- Battula VL, Shi Y, Evans KW, et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J Clin Invest 2012;122:2066-78. [Crossref] [PubMed]
- Yeh SC, Wang PY, Lou YW, et al. Glycolipid GD3 and GD3 synthase are key drivers for glioblastoma stem cells and tumorigenicity. Proc Natl Acad Sci U S A 2016;113:5592-7. [Crossref] [PubMed]
- Iwasawa T, Zhang P, Ohkawa Y, et al. Enhancement of malignant properties of human glioma cells by ganglioside GD3/GD2. Int J Oncol 2018;52:1255-66. [PubMed]
- Okada M, Furukawa K, Yamashiro S, et al. High expression of ganglioside alpha-2,8-sialyl- transferase (GD3 synthase) gene in adult T-cell leukemia cells unrelated to the gene expression of human T-lymphotropic virus type I. Cancer Res 1996;56:2844-8. [PubMed]
- Villanueva-Cabello TM, Mollicone R, Cruz-Muñoz ME, et al. Activation of human naïve Th cells increases surface expression of GD3 and induces neoexpression of GD2 that colocalize with TCR clusters. Glycobiology 2015;25:1454-64. [Crossref] [PubMed]
- Okamoto S, Mineno J, Ikeda H, et al. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res 2009;69:9003-11. [Crossref] [PubMed]


