
Massively parallel sequencing of urinary DNA—the dawn of non-invasive bladder cancer detection and surveillance?
Introduction
The recent paper by Dudley et al. (1) entitled “Detection and surveillance of bladder cancer using urine tumor DNA” adds to the burgeoning evidence that massively-parallel sequencing of urinary DNA reliably detects tumour-associated mutations in urinary DNA. This approach promises to resolve the long overdue need for a sensitive and specific non-invasive test for bladder cancer. In this editorial we discuss the existing body of evidence and what the study by Dudley et al. contributes to this rapidly-evolving field of cancer research.
Background
The development of a non-invasive detection test for bladder cancer to reduce reliance on cystoscopy is a high priority for clinicians and patients alike (2). Many such tests have been proposed over the years, mostly based on increased levels of specific proteins in urine; however, none have been widely adopted due to a lack of sensitivity and/or specificity and a lack of high-quality evidence (3). Over the last decade massively parallel or “next generation sequencing” (NGS) has been used to characterise the genomic changes that are observed in large cohorts of bladder tumours (4). NGS serves not only as a discovery tool revealing new biomarkers for bladder cancer, but can also be exploited to detect these biomarkers in urine at very low levels of tumour DNA. NGS can be used to determine DNA methylation and copy number changes and to detect somatic mutations (SMs) such as insertions and deletions (indels) and single nucleotide variants (SNVs) in urinary DNA (5-13). Applied appropriately, targeted NGS has the ability to determine the presence of multiple SMs at low mutant allele frequencies (MAF), as may be the case in urine where tumour DNA often only comprises a small proportion of the total DNA present. SMs can also be analysed by a range of other techniques, but analogue methods have limited ability to detect SMs at low MAFs, and although ddPCR can detect ultra-low MAFs, it cannot be highly multiplexed to efficiently measure large numbers of SMs.
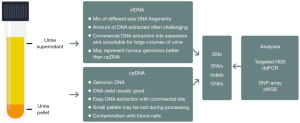
The use of NGS to detect SMs in single genes was initially demonstrated for FGR3 by Millholland et al. and the TERT promotor by Kinde et al. (7,8). Subsequently, Ward et al. used a multiplex-PCR approach to sequence hotspots in a panel of 8 bladder cancer genes in 121 bladder cancers of mixed grades and stages, and Scott et al. used a capture-based method to sequence 341 genes in the urine of HR-NMIBC patients treated with BCG (9,10). These studies all used DNA extracted from cells present in the urine. Two non-NGS-based studies suggested that urinary cell-free DNA (cfDNA) may better recapitulate tumour genomic changes than urine cell-pellet DNA (cpDNA) (6,14); however, recent NGS-based studies have reported comparable performances using cfDNA and cpDNA for detecting residual/recurrent disease in MIBC patients receiving neoadjuvant chemotherapy using an 8-gene panel (11), and for detecting TERT promoter mutations across stages and grades of incident UBC (12). Seemingly, cfDNA or cpDNA can be used interchangeably if one or other DNA preparation fails, or can be used to confirm results from one another when both are available (Figure 1). What is clear is that a panel of carefully selected SMs must be analysed with a method that is able to detect very low MAFs with very low error rates in order to achieve both high sensitivity and specificity. Providing that there are few false-positives due to sequencing errors, cancer-associated SMs should provide a very specific route to the detection of UBC [although SMs can occur in some other bladder lesions such as inverted papillomas (15)]. Additionally, pre-malignant changes and microscopic residual disease may have to be considered, particularly in the surveillance setting.
The largest targeted NGS analysis of SMs in urinary DNA to date is the study by Springer et al. (13). In this study, hotspots were targeted in 9 UBC-associated genes and 2 kidney cancer genes as well as genome-wide aneuploidy. The study used cpDNA and PCR-based library preparation incorporating unique molecular identifiers (UMIs) to allow SNV detection as low as 0.03% MAF. This small gene panel identified SNVs in 89 out of 102 UBCs and thus has a maximum theoretical sensitivity of 89%. Whilst the inclusion of aneuploidy should increase sensitivity further, aneuploidy detection requires a much higher fraction of the urinary DNA to be derived from tumour cells than does SNV detection. In a cohort of 570 patients undergoing investigation for incident UBC, the test performed with a sensitivity of 83% at a specificity of 93%. The test also detected 42 out of 56 upper tract cancers (75% sensitivity), and in a cohort of UBC patients undergoing surveillance performed with a sensitivity of 68% at a specificity of 80%. Although the Springer study utilised an assay with very high analytical sensitivity, disease detection sensitivity was limited (partially) by the size of the gene panel and choice of genes in the panel. It is probable that a more extensive gene panel might improve sensitivity, and using cfDNA rather than cpDNA might also improve test performance. It is these two questions that the study by Dudley et al. addresses.
The study
Dudley et al. use a capture-based library preparation method to sequence c.311 kB of DNA across 460 genes in urinary cfDNA from 118 UBC patients and 67 healthy adults. The panel was initially applied to 60 UBC tumour specimens detecting a median of 6 SMs per tumour and, according to Table S5, ≥1 SNV in 57 of the 60 tumours, equivalent to a maximum theoretical sensitivity of 95%. A major finding in the tumour data is that SNVs in the PLEKHS1 promoter occurred in 26/60 tumour samples (43%), making this the second most common mutation site in UBC. This biomarker, included based on the pan-cancer analysis of mutations in regulatory regions by Weinhold et al. (16), has not previously been included in urinary NGS studies but is extremely likely to be an important constituent of a SM-based diagnostic test for UBC. Additional useful technical information for the field is also provided: an economical and effective way to extract cfDNA from large volumes of urine is presented, evidence that size selection of urinary cfDNA is not necessary, that EDTA effectively stabilises urinary cfDNA and that enzymatic fragmentation of urinary cfDNA is superior to cleavage by ultrasonic shearing.
For 18 patients, paired tumour tissue and urine were analysed; 66.7% of the mutations detected in the tumours were also detected in the paired cfDNA. Concordance was particularly high for putative driver mutations and mutations with higher MAFs in the tissue (presumably truncal). Two data analysis approaches were subsequently used to determine sensitivity for UBC detection via urinary cfDNA: “tumour-informed” and “tumour-naïve”, with thresholds for variant calling established using cfDNA from 33 young healthy controls. The tumour-informed approach only considered SMs present in the index tumour and used Monte Carlo P value thresholds for variant detection. The tumour-informed approach is potentially applicable in the post-TURBT surveillance setting, but not in the initial stages of UBC detection, e.g., in haematuria clinic. The tumour naïve approach considered OncoKB “oncogenic” SNVs, TERT & PLEKHS1 promotor SNVs, truncating mutations in tumour suppressors and CNVs using 0.5% MAF as the threshold for SNV detection. The 2 data analysis approaches were applied to 54 patients with biopsy-proven incident UBC and 34 non-UBC controls. The tumour-naïve approach achieved 83% sensitivity (72% for low-grade UBC and 96% for high-grade UBC) at 97% specificity. The tumour-informed approach was applied to 27 of the UBC patients and achieved a sensitivity of 93% at 96% specificity. The sensitivity and specificity for the tumour-naïve approach on these 27 UBC patients are not provided for comparison. Unsurprisingly, both versions of the SM-based test considerably outperform urine cytology.
SM-based UBC detection was also tested in the surveillance setting using urinary cfDNA from 37 patients that subsequently developed recurrence, and 27 patients that were recurrence-free for at least 9 months following urine collection. The tumour-naïve approach yielded a sensitivity of 84% at 96% specificity, and the tumour-informed approach (applied to only 22 UBC patients with tissue available) yielded a sensitivity of 91% at 100% specificity. Detection of SMs in urinary cfDNA preceded clinically detected recurrence by 2.7 months. Although this lead-time seems quite plausible, the samples were selected from a much larger cohort of patients (n=420) on the basis that they were the earliest samples available for patients whom ultimately experienced disease recurrence (detected by cystoscopy), thus biasing against cystoscopy. Additionally, the sample sizes in the 2 arms of this study are small and 95% confidence intervals on sensitivity and specificity, although not presented, will be wide. Nonetheless, the performances of Dudley et al.’s SM-based UBC test in both the incident disease and surveillance settings are impressive and warrant validation in large-scale studies. Furthermore, the accurate analysis of tumour SMs in a urine sample may permit the near real-time monitoring of tumour evolution during intravesical therapy, neoadjuvant chemotherapy or chemoradiotherapy, and the potential to adjust therapeutic approaches (17).
SMs are identified in less than half of the 460 genes in the panel used by Dudley et al., and our experience with SM detection in urinary DNA suggests that high sensitivity may be achieved using tens of carefully selected genes rather than hundreds of genes (manuscript in preparation). However, both our unpublished findings and data published by Springer et al. (13) suggest that detecting SMs with extremely low MAFs (<0.5%) is essential for detecting UBC with high sensitivity. We suggest that selectively “trimming” the gene panel and incorporating UMIs to lower SM calling thresholds might improve Dudley et al.’s test even further. Additionally, the study does not compare urinary cfDNA with cpDNA, and similar sensitivity and specificity might also be achievable with the latter [which is both easier to extract and more abundant (18)].
Conclusions
In summary, the study by Dudley et al. is an impressive demonstration of the utility of SM detection in urinary cfDNA for non-invasive UBC detection. It represents another example of the use of urinary DNA NGS to detect SMs at low MAFs, and perhaps heralds the dawn of non-invasive testing for UBC. Large-scale studies and clinical trials are awaited in order to translate these and similar findings for the benefit of UBC patients, endeavours that could lead to one of the biggest changes in urological practice for over half a century.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Peng Zhang, MD, PhD (Department of Urology, Zhongnan Hospital of Wuhan University, Wuhan, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.03.03). RT Bryan has contributed to advisory boards for Olympus Medical Systems with regard to narrow band imaging cystoscopy. DG Ward has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dudley JC, Schroers-Martin J, Lazzareschi DV, et al. Detection and Surveillance of Bladder Cancer Using Urine Tumor DNA. Cancer Discov 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Bessa A, Maclennan S, Enting D, et al. Consensus in Bladder Cancer Research Priorities Between Patients and Healthcare Professionals Using a Four-stage Modified Delphi Method. Eur Urol 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Schmitz-Dräger BJ, Droller M, Lokeshwar VB, et al. Molecular markers for bladder cancer screening, early diagnosis, and surveillance: the WHO/ICUD consensus. Urol Int 2015;94:1-24. [Crossref] [PubMed]
- Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2018;174:1033. [Crossref] [PubMed]
- Feber A, Dhami P, Dong L, et al. UroMark-a urinary biomarker assay for the detection of bladder cancer. Clin Epigenetics 2017;9:8. [Crossref] [PubMed]
- Togneri FS, Ward DG, Foster JM, et al. Genomic complexity of urothelial bladder cancer revealed in urinary cfDNA. Eur J Hum Genet 2016;24:1167-74. [Crossref] [PubMed]
- Kinde I, Wu J, Papadopoulos N, et al. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci U S A 2011;108:9530-5. [Crossref] [PubMed]
- Millholland JM, Li S, Fernandez CA, et al. Detection of low frequency FGFR3 mutations in the urine of bladder cancer patients using next-generation deep sequencing. Res Rep Urol 2012;4:33-40. [PubMed]
- Scott SN, Ostrovnaya I, Lin CM, et al. Next-generation sequencing of urine specimens: A novel platform for genomic analysis in patients with non-muscle-invasive urothelial carcinoma treated with bacille Calmette-Guérin. Cancer Cytopathol 2017;125:416-26. [Crossref] [PubMed]
- Ward DG, Baxter L, Gordon NS, et al. Multiplex PCR and Next Generation Sequencing for the Non-Invasive Detection of Bladder Cancer. PLoS One 2016;11:e0149756. [Crossref] [PubMed]
- Patel KM, van der Vos KE, Smith CG, et al. Association Of Plasma And Urinary Mutant DNA With Clinical Outcomes In Muscle Invasive Bladder Cancer. Sci Rep 2017;7:5554. [Crossref] [PubMed]
- Stasik S, Salomo K, Heberling U, et al. Evaluation of TERT promoter mutations in urinary cell-free DNA and sediment DNA for detection of bladder cancer. Clin Biochem 2019;64:60-3. [Crossref] [PubMed]
- Springer SU, Chen CH, Rodriguez Pena MDC, et al. Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. Elife 2018;7.
- Szarvas T, Kovalszky I, Bedi K, et al. Deletion analysis of tumor and urinary DNA to detect bladder cancer: urine supernatant versus urine sediment. Oncol Rep 2007;18:405-9. [PubMed]
- McDaniel AS, Zhai Y, Cho KR, et al. HRAS mutations are frequent in inverted urothelial neoplasms. Hum Pathol 2014;45:1957-65. [Crossref] [PubMed]
- Weinhold N, Jacobsen A, Schultz N, et al. Genome-wide analysis of noncoding regulatory mutations in cancer. Nat Genet 2014;46:1160-5. [Crossref] [PubMed]
- Ward DG, Bryan RT. Liquid biopsies for bladder cancer. Transl Androl Urol 2017;6:331-5. [Crossref] [PubMed]
- Russo IJ, Ju Y, Gordon NS, et al. Toward Personalised Liquid Biopsies for Urothelial Carcinoma: Characterisation of ddPCR and Urinary cfDNA for the Detection of the TERT 228 G>A/T Mutation. Bladder Cancer 2018;4:41-8. [Crossref] [PubMed]


