
MiR-451 suppresses the growth, migration, and invasion of prostate cancer cells by targeting macrophage migration inhibitory factor
Introduction
Metastasis is the most common cause of death of prostate cancer patients. The mechanisms underlying prostate cancer metastasis is not completely clear. Therefore, identification of key regulators with functional role in the suppression or enhancement of prostate cancer metastasis is essential to improve patients’ prognosis and disease management. miRNAs are small non-coding RNAs that can negatively regulate the expression of target genes by binding to their 3’ untranslated regions (UTRs) and promoting mRNA degradation or inhibiting translation (1,2). Many studies have demonstrated that miRNAs are dysregulated in prostate cancers suggesting their roles in cancer onset, cell invasion, migration, and metastasis. The altered miR-451 expression has been reported in various types of tumors. However, the functions of miR-451 in different types of tumors are not consistent. For example, it has been reported that miR-451 can inhibit cancer cell proliferation and invasion and its downregulation is associated with a poor patient outcome in gastric, lung, renal, liver and bladder cancers (3-7); oppositely, in pancreatic and thyroid cancer, an increased expression of miR-451 has been reported to promote cell proliferation and metastasis (8,9). The role of miR-451 in prostate cancer has not been reported so far. In this study, we examined the expression of miR-451 in clinical metastatic and primary localized prostate cancer tissues and prostate cancer cell lines with various metastatic abilities, confirmed a direct target of miR-451, and investigated the function of miR-451 in prostate cancer cell growth, migration, and invasion.
Methods
Clinical samples
Fresh prostate cancer samples were collected from the 2nd Hospital of Tianjin Medical University and Tianjin Institute of Urology including 31 benign prostates, 29 localized primary cancer tissues harvested from radical prostatectomy and 12 lymph node metastasis tissues. The cancer specimens were immediately frozen in liquid nitrogen after surgery and were stored in −80 °C freezer. This study was approved by the research ethics committee of the Second Hospital of Tianjin Medical University. Clinical information of these samples was provided in Table S1.
Cell culture
LNCaP, C4-2, PC3, and PC3M cells were cultured in an RPMI-1640 medium (Thermo Fisher Scientific, Shanghai, China) containing 10% fetal bovine serum (Thermo Fisher Scientific) at 37 °C with 5% CO2.
Real-time PCR
The total RNA was extracted from cultured cells and clinical prostate cancer tissues using TRIzol reagent (Thermo Fisher Scientific) followed the manufacturer’s instructions. Final RNA concentration was adjusted to 100 ng/µL and 1ug of RNA was used for reverse transcription reaction. The complementary DNA (cDNA) was synthesized using a TaqMan MicroRNA Reverse Transcription Kit and miRNA-specific RT primers from a TaqMan MicroRNA Assay (Thermo Fisher Scientific). The expression of miR-451 was measured by TaqMan miRNA assays in accordance with the manufacturer’s instructions (Thermo Fisher Scientific). The miR-451 expression in all the samples was normalized to U6 small nuclear RNA. To exam the expression of migration inhibitory factor (MIF), total RNA was isolated from cultured cells or clinical samples using the RNeasy mini kit (Thermo Fisher Scientific) following the manufacturer’s instructions. cDNAs were synthesized using a QuantiTect Reverse Transcription Kit (Qiagen Inc.). qRT-PCR reactions using SYBR Green Premix Ex Taq (TaKaRa, Dalian, China) were performed in a ViiA 7 Real-Time PCR system (Applied Biosystems, Thermo Fisher Scientific).
Transfection
miR-451 mimics or scramble were synthesized by Sangon Biotech Co., Ltd., Shanghai, China. In a ‘rescue’ experiment, MIF coding sequence without its 3'-UTR was cloned into a pcDNA3 vector (Thermo Fisher Scientific). The miR-451 mimics or scramble or pc-MIF vector were transfected into C4-2 or PC3M cells together or separately for 48 hours by Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. The final concentration of mimics and scramble RNA is 25 nM.
Western blotting
The culture medium was removed from the culture dish and the cells were rinsed with PBS gently. RIPA lysis buffer was added into the dishes to prepare the total protein of cells. The protein concentration was measured using the BCA protein assay (Beyotime Institute of Biotechnology, Shanghai, China). The protein concentration was adjusted to 1 µg/µL and 10 µg of protein was loaded per lane. The Anti-MIF antibody (R&D Systems) was used for detecting MIF expression and an anti-Actin antibody (Sigma-Aldrich) was used for detecting actin as the loading control. Immunoblotting signals were detected using the Super Signal West Pico Chemiluminescent Substrate Kit (Pierce Biotechnology).
Luciferase reporter gene assay
The 3'-UTR sequence of MIF predicted to interact with miR-451 and a mutant 3'-UTR sequence were synthesized and cloned into psiCHECK-2 vector (Promega). miR-451 mimics or scramble sequence was co-transfected into C4-2 cells with the plasmid DNAs using lipofectamine 2000. Luciferase activates were determined using the Dual-Luciferase Assay System (Promega) after 48 hours of transfection. Renilla luciferase activities were normalized to the internal control Firefly luciferase activity.
Cell migration assay and invasion assay
Uncoated transwell chambers and modified Boyden chambers (BD Biosciences) with 8 µm pore size were used to examine the cell migration and invasion ability respectively. In brief, C4-2 or PC3M cells were suspended in serum-free medium and were seeded to the upper chambers. The medium containing 5% fetal bovine serum was added to the lower chambers. The cells and chambers were incubated at 37 °C for 22 hours. After incubation, cells that were adhered to the upper surface of the chamber were wiped off. The cells on the lower surface were fixed, stained with crystal violet and counted under a light microscope.
Statistical analysis
Data are presented as mean ± SD. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software). Two-tailed Student’s t-test was used for comparisons between every two groups in all relevant experiments. The relationship between MIF and miR-451 expression was assessed using Pearson’s correlation analysis. The significance threshold is set at P<0.05 to indicate statistical significance.
Results
Decreased expression of miR-451 was observed in clinical metastatic prostate cancer tissues and the metastatic prostate cancer cell lines
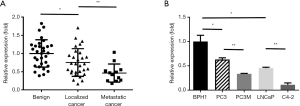
To investigate the miR-451 expression in prostate cancer, we first examined its expression in 31 benign prostates, 29 localized primary prostate cancer, and 12 lymph node metastatic prostate cancer specimens. The qRT-PCR results demonstrated that the expression of miR-451 in lymph node metastatic prostate cancer tissues was significantly lower than that in localized prostate cancer and benign prostate tissues (P<0.01), which indicates an association of miR-451 expression with prostate cancer metastasis (Figure 1A). Furthermore, we examined miR-451 expression in a panel of cell lines derived from benign prostate or prostate cancer including a benign prostate epithelial cell line (BPH-1), prostate cancer cell lines LNCaP, PC3 and their highly metastatic derivatives C4-2 and PC3M. We observed that all the cancer cell lines showed a significant decrease of miR-451 expression compared to benign BPH1 cell line. Interestingly, highly metastatic C-4 and PC3M cell line showed less miR-451 expression than low-metastatic LNCaP and PC3 cells (P<0.01) (Figure 1B).
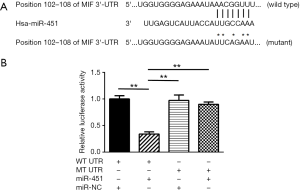
MiR-451 directly targets MIF in prostate cancer
To have a better understanding of the mechanisms underlying miR-451 regulated prostate cancer metastasis, we identified MIF as a potential target gene of miR-451 using TargetScan Release 7.0 (Figure 2A). To validate MIF is a direct target gene of miR-451, we used a luciferase reporter assay by cloning the wild-type MIF 3'-UTR sequence or a mutant one (Figure 2A) into a psiCHECK-2 vector and co-transfecting the vector accompany with miR-451 mimics or scramble sequence into C4-2 cells. Transfection of miR-451 mimics significantly inhibited the luciferase activity of reporter with wild-type 3'-UTR of MIF (P<0.01), but not that with the mutant 3'-UTR. As expected, transfection of scramble sequence didn’t inhibit the activity of luciferase of the reporter with wild-type 3'-UTR of MIF (Figure 2B).
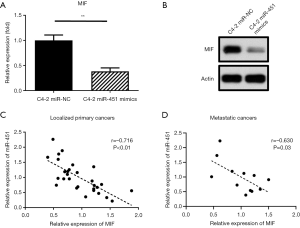
Consistent with the luciferase assay results, the mRNA and protein expression of MIF in miR-451-overexpressed cells was apparently decreased compared to those of the cells transfected with scramble sequence (Figure 3A,B). Furthermore, analysis of RNA expression data of clinical prostate cancer samples showed a strong negative correlation between the expression of miR-451 and MIF, supporting MIF is a direct target of miR-451 in prostate cancer (Figure 3C,D).
MiR-451 suppresses prostate cancer cell growth, migration, and invasion
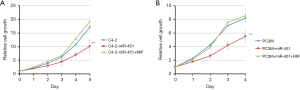
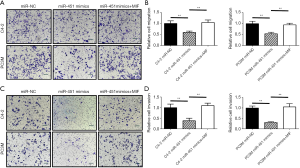
Following the observation of decreased miR-451 expression in highly metastatic C4-2 and PC3M cells, we subsequently utilized these cell lines to study the function of miR-451 in prostate cancer. We transfected the mimics of miR-451 or scramble sequence in C4-2 and PC3M cells respectively, then examined the effect of miR-451 on prostate cancer viability and proliferation using MTT assay. We observed that cells transfected with miR-451 mimics presented a significant growth inhibition compared with those transfected with scramble miR (P<0.01, Figure 4A,B). Transwell migration assay showed that the migration ability of C4-2 and PC3M cells transfected with miR-451 mimics was significantly decreased compared to the cells transfected with scramble controls (Figure 5A,B). Consistently, the Matrigel (Boyden chamber) invasive assay demonstrated overexpression of miR-451 significantly suppressed C4-2 and PC3M cell invasion (P<0.01, Figure 5C,D). Moreover, a ‘rescue’ experiment was performed by overexpressing MIF in C4-2-miR-451 and PC3M-miR-451 cells. The overexpression of MIF significantly reversed the suppression effects of cell proliferation, migration and invasion induced by miRNA-451 (P<0.01, Figure 5A,B,C,D). These results suggest that miR-451 suppresses prostate cancer cell progression via directly regulating MIF expression.
Discussion
Most deaths caused by prostate cancer are related to tumor metastases that are highly resistant to the treatment. However, the mechanisms underlying prostate cancer metastasis is not completely understood. MicroRNAs, small RNA molecules that regulate gene expression post-transcriptionally, have been reported to play a key role in cancer development and progression (10). miRNA-451 was observed to be deregulated and act as oncogene or tumor suppressor miRNA in various types of cancers, suggesting a context-dependent effect. However, its role in prostate cancer is not clear.
In this study, we observed the downregulation of miR-451 in metastatic prostate cancer tissues compared to localized primary prostate cancer and benign tissues. Functionally, we demonstrated an increased miR-451 expression can significantly suppress prostate cancer cell proliferation, migration, and invasion, which suggests miR-451 plays a metastasis-suppressing role in prostate cancer. These results suggest miR-451 may be a potential biomarker for metastatic prostate cancer. One major limitation of this study is small clinical sample size used for clinical relevance study. Further evaluation of the prognostic value of miR-451 in prostate cancer using large clinical cohorts in the future will provide more insight to its potential application in outcome prediction of prostate cancer patients. Recently, it was reported that low expression of miR-451 was significantly correlated with short progression-free survival (PFS) and overall survival (OS), and miR-451 was an independent poor prognostic factor for PFS and OS in metastatic castration resistant prostate cancer patients (11) and its increased expression is associated with hypoxia in prostate cancer cells (12). Further validation expression of miR-451 in plasma or serum of prostate cancer patients will provide more evidence for its value in the detection of metastatic prostate cancer, monitoring of treatment response, and guiding personalized therapy.
A few target genes of miR-451 have been reported in different types of cancer, such as CAB39, RAB14, 14-3-3§ and MIF, have been identified (13-15). MIF is a proinflammatory cytokine and its increased expression has been observed in various types of tumors, such as lung, colorectal, breast, cervical, prostate, and head and neck cancer (16). The increased MIF expression plays a crucial role in cell proliferation, invasiveness and tumor-induced angiogenesis (17-21). The inhibition of MIF or its receptor (CD74) can attenuate prostate cancer cell growth and invasion (22,23). In our study, we proved the direct binding of miR-451 and 3'-UTR of MIF in prostate cancer cells. We also further confirmed that MIF protein expression was decreased in C4-2 prostate cancer cells transfected with miR-451 mimics compared with the cells transfected with scramble sequence by Western blotting analyses. Moreover, we observed a strong negative correlation between the expression of miR-451 and MIF. Functionally, overexpression of MIF in C4-2 and PC3M prostate cancer cells transfected with miR-451 mimics can reverse the effect of miR-451. These data suggested that miR-451 may directly control MIF protein expression and further regulate cell growth, migration, and invasion in prostate cancer. Our results are consistent with the findings reported in gastric cancer and nasopharyngeal carcinoma (24,25).
In conclusion, this study demonstrated that miR-451 is downregulated in prostate cancer, especially in metastatic prostate cancer. MiR-451 can functionally suppress prostate cancer cell growth, migration and invasion by directly targeting MIF. These findings provide insight into the development of new therapeutic approaches for prostate cancer treatment.
Table S1
| Sample ID | Age at diagnosis | Tissue source | Gleason score | T stage |
|---|---|---|---|---|
| 1 | 70 | Benign | N/A | N/A |
| 2 | 60 | Benign | N/A | N/A |
| 3 | 78 | Benign | N/A | N/A |
| 4 | 58 | Benign | N/A | N/A |
| 5 | 66 | Benign | N/A | N/A |
| 6 | 72 | Benign | N/A | N/A |
| 7 | 66 | Benign | N/A | N/A |
| 8 | 70 | Benign | N/A | N/A |
| 9 | 67 | Benign | N/A | N/A |
| 10 | 65 | Benign | N/A | N/A |
| 11 | 70 | Benign | N/A | N/A |
| 12 | 75 | Benign | N/A | N/A |
| 13 | 70 | Benign | N/A | N/A |
| 14 | 62 | Benign | N/A | N/A |
| 15 | 63 | Benign | N/A | N/A |
| 16 | 70 | Benign | N/A | N/A |
| 17 | 72 | Benign | N/A | N/A |
| 18 | 73 | Benign | N/A | N/A |
| 19 | 63 | Benign | N/A | N/A |
| 20 | 65 | Benign | N/A | N/A |
| 21 | 70 | Benign | N/A | N/A |
| 22 | 65 | Benign | N/A | N/A |
| 23 | 68 | Benign | N/A | N/A |
| 24 | 70 | Benign | N/A | N/A |
| 25 | 74 | Benign | N/A | N/A |
| 26 | 64 | Benign | N/A | N/A |
| 27 | 56 | Benign | N/A | N/A |
| 28 | 61 | Benign | N/A | N/A |
| 29 | 67 | Benign | N/A | N/A |
| 30 | 64 | Benign | N/A | N/A |
| 31 | 59 | Benign | N/A | N/A |
| 32 | 71 | Localized prostate adenocarcinoma | 3+4 | 2a |
| 33 | 74 | Localized prostate adenocarcinoma | 3+4 | 2b |
| 34 | 73 | Localized prostate adenocarcinoma | 3+5 | 3 |
| 35 | 78 | Localized prostate adenocarcinoma | 3+4 | 2c |
| 36 | 64 | Localized prostate adenocarcinoma | 4+3 | 2b |
| 37 | 74 | Localized prostate adenocarcinoma | 4+5 | 2c |
| 38 | 58 | Localized prostate adenocarcinoma | 4+4 | 2a |
| 39 | 63 | Localized prostate adenocarcinoma | 3+4 | 2b |
| 40 | 65 | Localized prostate adenocarcinoma | 4+3 | 3 |
| 41 | 66 | Localized prostate adenocarcinoma | 3+4 | 2c |
| 42 | 63 | Localized prostate adenocarcinoma | 4+4 | 2b |
| 43 | 68 | Localized prostate adenocarcinoma | 4+4 | 2c |
| 44 | 70 | Localized prostate adenocarcinoma | 5+4 | 3 |
| 45 | 65 | Localized prostate adenocarcinoma | 3+4 | 3 |
| 46 | 70 | Localized prostate adenocarcinoma | 4+5 | 2b |
| 47 | 73 | Localized prostate adenocarcinoma | 3+4 | 2c |
| 48 | 61 | Localized prostate adenocarcinoma | 4+4 | 2c |
| 49 | 65 | Localized prostate adenocarcinoma | 4+5 | 3 |
| 50 | 63 | Localized prostate adenocarcinoma | 4+4 | 2b |
| 51 | 77 | Localized prostate adenocarcinoma | 3+4 | 2b |
| 52 | 66 | Localized prostate adenocarcinoma | 3+4 | 2c |
| 53 | 67 | Localized prostate adenocarcinoma | 4+4 | 3 |
| 54 | 72 | Localized prostate adenocarcinoma | 3+4 | 2a |
| 55 | 67 | Localized prostate adenocarcinoma | 3+3 | 2b |
| 56 | 73 | Localized prostate adenocarcinoma | 4+4 | 2c |
| 57 | 60 | Localized prostate adenocarcinoma | 5+4 | 2c |
| 58 | 64 | Localized prostate adenocarcinoma | 3+4 | 2b |
| 59 | 56 | Localized prostate adenocarcinoma | 3+3 | 2c |
| 60 | 70 | Localized prostate adenocarcinoma | 4+4 | 2b |
| 61 | 68 | Lymph node metastasis | 4+4 | 2c |
| 62 | 65 | Lymph node metastasis | 3+4 | 3 |
| 63 | 61 | Lymph node metastasis | 4+4 | 2 |
| 64 | 61 | Lymph node metastasis | 4+5 | 3 |
| 65 | 76 | Lymph node metastasis | 5+4 | 2b |
| 66 | 73 | Lymph node metastasis | 4+4 | 2c |
| 67 | 60 | Lymph node metastasis | 5+4 | 2c |
| 68 | 70 | Lymph node metastasis | 4+4 | 2b |
| 69 | 65 | Lymph node metastasis | 4+3 | 3 |
| 70 | 66 | Lymph node metastasis | 4+4 | 2c |
| 71 | 73 | Lymph node metastasis | 5+4 | 3 |
| 72 | 61 | Lymph node metastasis | 5+4 | 3 |
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.03.28). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all patients. The study was approved by the research ethics committee of the Second Hospital of Tianjin Medical University (No. KY2018K090).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 2015;15:321-33. [Crossref] [PubMed]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009;10:704-14. [Crossref] [PubMed]
- Su Z, Zhao J, Rong Z, et al. MiR-451, a potential prognostic biomarker and tumor suppressor for gastric cancer. Int J Clin Exp Pathol 2015;8:9154-60. [PubMed]
- Goto A, Tanaka M, Yoshida M, et al. The low expression of miR-451 predicts a worse prognosis in non-small cell lung cancer cases. PLoS One 2017;12:e0181270. [Crossref] [PubMed]
- Zhu S, Huang Y, Su X. Mir-451 Correlates with Prognosis of Renal Cell Carcinoma Patients and Inhibits Cellular Proliferation of Renal Cell Carcinoma. Med Sci Monit 2016;22:183-90. [Crossref] [PubMed]
- Li HP, Zeng XC, Zhang B, et al. miR-451 inhibits cell proliferation in human hepatocellular carcinoma through direct suppression of IKK-beta. Carcinogenesis 2013;34:2443-51. [Crossref] [PubMed]
- Zeng T, Peng L, Chao C, et al. miR-451 inhibits invasion and proliferation of bladder cancer by regulating EMT. Int J Clin Exp Pathol 2014;7:7653-62. [PubMed]
- Guo R, Gu J, Zhang Z, et al. MiR-451 Promotes Cell Proliferation and Metastasis in Pancreatic Cancer through Targeting CAB39. Biomed Res Int 2017;2017:2381482. [Crossref] [PubMed]
- Wang Z, Zhang H, Zhang P, et al. Upregulation of miR-2861 and miR-451 expression in papillary thyroid carcinoma with lymph node metastasis. Med Oncol 2013;30:577. [Crossref] [PubMed]
- Sassen S, Miska EA, Caldas C. MicroRNA: implications for cancer. Virchows Arch 2008;452:1-10. [Crossref] [PubMed]
- Chen DQ, Yu C, Zhang XF, et al. HDAC3-mediated silencing of miR-451 decreases chemosensitivity of patients with metastatic castration-resistant prostate cancer by targeting NEDD9. Ther Adv Med Oncol 2018;10:1758835918783132. [Crossref] [PubMed]
- Panigrahi GK, Ramteke A, Birks D, et al. Exosomal microRNA profiling to identify hypoxia-related biomarkers in prostate cancer. Oncotarget 2018;9:13894-910. [Crossref] [PubMed]
- Tian Y, Nan Y, Han L, et al. MicroRNA miR-451 downregulates the PI3K/AKT pathway through CAB39 in human glioma. Int J Oncol 2012;40:1105-12. [PubMed]
- Wang R, Wang ZX, Yang JS, et al. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14). Oncogene 2011;30:2644-58. [Crossref] [PubMed]
- Bergamaschi A, Katzenellenbogen BS. Tamoxifen downregulation of miR-451 increases 14-3-3zeta and promotes breast cancer cell survival and endocrine resistance. Oncogene 2012;31:39-47. [Crossref] [PubMed]
- Conroy H, Mawhinney L, Donnelly SC. Inflammation and cancer: macrophage migration inhibitory factor (MIF)--the potential missing link. QJM 2010;103:831-6. [Crossref] [PubMed]
- Rendon BE, Roger T, Teneng I, et al. Regulation of human lung adenocarcinoma cell migration and invasion by macrophage migration inhibitory factor. J Biol Chem 2007;282:29910-8. [Crossref] [PubMed]
- He XX, Chen K, Yang J, et al. Macrophage migration inhibitory factor promotes colorectal cancer. Mol Med 2009;15:1-10. [Crossref] [PubMed]
- Richard V, Kindt N, Decaestecker C, et al. Involvement of macrophage migration inhibitory factor and its receptor (CD74) in human breast cancer. Oncol Rep 2014;32:523-9. [Crossref] [PubMed]
- Guo P, Wang J, Liu J, et al. Macrophage immigration inhibitory factor promotes cell proliferation and inhibits apoptosis of cervical adenocarcinoma. Tumour Biol 2015;36:5095-102. [Crossref] [PubMed]
- Meyer-Siegler KL, Iczkowski KA, Leng L, et al. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol 2006;177:8730-9. [Crossref] [PubMed]
- Meyer-Siegler KL, Iczkowski KA, Vera PL. Further evidence for increased macrophage migration inhibitory factor expression in prostate cancer. BMC Cancer 2005;5:73. [Crossref] [PubMed]
- Meyer-Siegler K, Hudson PB. Enhanced expression of macrophage migration inhibitory factor in prostatic adenocarcinoma metastases. Urology 1996;48:448-52. [Crossref] [PubMed]
- Bandres E, Bitarte N, Arias F, et al. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res 2009;15:2281-90. [Crossref] [PubMed]
- Liu N, Jiang N, Guo R, et al. MiR-451 inhibits cell growth and invasion by targeting MIF and is associated with survival in nasopharyngeal carcinoma. Mol Cancer 2013;12:123. [Crossref] [PubMed]


