
Negative predictive value (NPV) of FDG PET-CT for nodal disease in clinically node-negative early stage lung cancer (AJCC 7th ed T1-2aN0) and identification of risk factors for occult nodal (pN1-N2) metastasis: implications for SBRT
Introduction
Lung cancer remains the leading cause of cancer death with an incidence, in 2010, of more than 220,000 in the USA (1). Despite advances in chemotherapy, radiation therapy, and surgery, prognosis remains poor with an overall 5-year survival of 14% (2). Correct clinical staging is key to successful management. In particular, correctly identifying patients with potentially curable non-small cell lung cancer (NSCLC) is a vital management goal (3,4). Furthermore, recent development of treatment strategies including stereotactic body radiation therapy (SBRT), sublobar resection, and radiofrequency ablation, where routine assessment of nodal disease may not be done, increases the importance of detecting the nodal spread of disease.
The introduction of modern imaging systems such as computed tomography (CT) and positron emission tomography-computed tomography (PET-CT) with fluorodeoxyglucose (FDG) has improved the detection of peripheral lung cancers at earlier stages (5-7). However, the performance of PET-CT for both mediastinal and lobar nodal staging remains an area of active investigation. A recently published meta-analysis of ten studies evaluating the ability of PET-CT to detect nodal disease reported a negative predictive value (NPV) for N1-N2 metastases of 87%. PET-CT performed less well for T2 primary tumors (8).
For early stage, node-negative NSCLCs surgical resection is considered the standard of care. In particular, lobectomy with lymphadenectomy remains the treatment of choice for early-stage cancers, regardless of tumor size or metabolic features on FDG PET-CT. Over the last decade, there has been a reassessment of sublobar resection and other approaches in the context of medically inoperable lung cancer. Preliminary results and less-invasive approaches have led to an investigation of these approaches in medically operable patients. Furthermore, the tumor, node, metastasis (TNM) classification system (7th edition) released in 2007 is a reflection of a trend suggesting small lung cancers may behave less aggressively and result in significantly longer survival relative to larger T1 disease (9). This suggests the potential for less aggressive surgical approaches to achieve cure while reducing morbidity (9-11). PET-CT is widely accepted as the best modality for preoperative clinical determination of disease stage and is essential for appropriate selection of candidates for non-systemic treatment.
The objective of this series is to assess the incidence of pN1-pN2 among those clinically staged with PET-CT as cN0 lung cancer and to identify predictors of nodal disease occult to PET-CT. Such predictors of occult nodal involvement could help establish criteria for patient selection in which radical lymphadenectomy might be necessary. Secondly, we sought to evaluate the accuracy of negative nodal uptake on PET-CT by comparing the performance of FDG PET-CT clinical staging to the true pathologic stage.
Methods
After obtaining IRB approval, the records of all patients who underwent surgical resection of pathologically confirmed NSCLC at the Norris Cotton Cancer Center/Dartmouth-Hitchcock Medical Center over a 3-year period were retrospectively reviewed. From that cohort, patients with AJCC/UICC 7th edition clinical stage IA and IB (T2a or less, N0, M0) based on pre-treatment integrated PET-CT were included in this analysis. Patients who had undergone neo-adjuvant therapy or who had staging PET-CT at an outside facility were excluded. The clinical stage I patients in this analysis did not undergo a separate mediastinal staging procedure. Mediastinal staging was done as part of the definitive surgery.
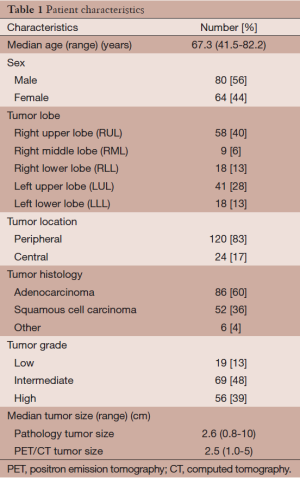
Patient age, lobar location, surgical pathology [adenocarcinoma, squamous cell carcinoma, other (large cell, bronchioloalveolar carcinoma)], tumor size at pathologic dissection, 7th edition AJCC/UICC TNM pathological stages, 7th edition AJCC/UICC TNM clinical stage based on PET-CT, and tumor size on PET-CT were recorded. CT images were re-reviewed to define the location of the primary tumor as central or peripheral. As in Al-Sarraf et al., a tumor was considered to be central if the center of the tumor lay in the inner 1/3 of the lung parenchyma on transverse CT images (12).
CT scans were acquired at one breath-hold at maximum inspiration during the injection of a 100 cm3 bolus of intravenous iodine contrast from the lung apices to the abdomen. CT examination was acquired using a standard 64-sice multidetector CTE (MDCT) protocol. Non-contrast enhanced PET-CT scans (Discovery ST, GE Healthcare) were acquired approximately 60 minutes following the intravenous administration of a standard dose of 18F-FDG (average 296-555 MBq). All positive nodes were localized using the American Thoracic Society (ATS) guidelines (13).
On PET-CT nodal FDG activity was compared to mediastinal blood pool activity to assess for increased uptake. Scans with activity not above mediastinal blood pool are considered negative (clinically N0).
Therefore nodules should not be compared to surrounding aerated lung, but rather to other solid soft tissue to assess for relatively increased uptake. Comparison typically can be made with mediastinal soft tissues or blood pool.
Though many institutions report SUV, give the well-known limitations of SUV, SUV is commonly but not routinely reported for FDG PET at our institution. We use the “not above mediastinal blood pool activity” as our cut-off for a negative scan.
Statistical analyses were conducted with Stata v11.0 (College Station, TX). The data were summarized using descriptive statistics (median and ranges for continuous variables, means and standard error for proportions). The frequency of agreement between the 7th edition AJCC/UICC clinical stage based on pre-treatment PET-CT and true stage based on pathologic dissection was calculated. Multivariate analysis was conducted using logistic regression with backward elimination. For multivariate logistic regression, multicollinearity was assessed with calculation of variance inflation factors (VIF, VIF >10 indicating multicollinearity).
Results
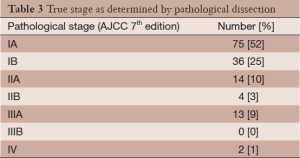
Over a three-year period, 144 patients with 7th edition AJCC/UICC stage IA and IB based on PET-CT underwent surgical resection at our center. Patient characteristics are presented in Table 1. Patients had pre-treatment staging PET-CT a median of 24 days prior to definitive surgery. Based on 7th edition AJCC/UICC staging, 98 patients were stage IA and 46 were stage IB as determined by PET-CT (Table 2). Of the 144 patients in this series, 91 (63%) were precisely correctly staged with PET-CT compared to the final pathologic stage: 71% correct for clinical stage IA, 46% correct for clinical stage IB. The majority of the other 53 patients not correctly staged by PET-CT (n=28) were upstaged to the next higher T category. The complete true pathological staging of all patients is presented in Table 3.
Full table

Full table
Full table
PET-CT NPV and pathological upstaging due to pN1-N2
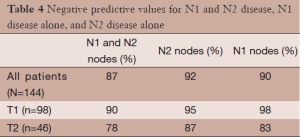
Of the 144 patients in this series clinically staged as IA or IB by PET-CT, 19 patients (9 of 98 clinical stage IAs and 10 of 46 clinical stage IBs) were upstaged at pathological dissection due to the presence of nodal metastases. Therefore, the overall NPV for nodal disease in this series is 87% (125/144). Of these 19 with nodal disease, 8 had N1 disease alone and 11 had N2 positive nodes. Six of these 11 patients with occult N2 disease, also had occult N1 positive nodes. The NPVs of PET-CT were 92% for N2 (mediastinal) disease (133/144) and 90% for N1 disease (130/144). The NPVs for mediastinal metastases were 95% in T1 disease (93/98) and 87% in T2 disease (40/46). The NPVs are displayed by stage and nodal status in Table 4.
Full table
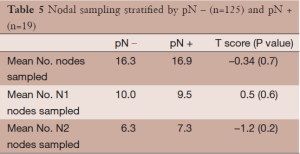
The average numbers of lymph nodes sampled (N1 and N2) are presented in Table 5. There was no statistically significant difference in the average total number of lymph nodes, the average number of N1 nodes, or the average number of N2 lymph nodes sampled between patients found to have positive lymph nodes on pathological dissection and those who were pathologically node negative.
Full table
Predictors of occult nodal disease
Multivariate logistic regression analysis for factors associated with occult nodal disease is summarized in Table 6. Backward elimination of non-significant factors produced a model containing tumor size (cm), central tumor location, and age at resection (Table 6). The categories of the tumor size variable were defined a priori according to the tumor size cutoffs in the 7th edition AJCC/UICC staging system. Multivariate logistic regression demonstrated a 3.28-fold increase in the risk of occult nodal disease for each higher T category (from T1A-2B) in the 7th edition AJCC/UICC staging system (adjusted OR: 3.28, 95% CI: 1.41-7.57). Of the 55 patients in this series with tumor diameter ≤2.0 cm, two were found to have occult nodal disease. However, 7 of 43 patients (16%) with tumor diameters between 2 and 3 cm and 10 of 46 patients (22%) with tumor diameter greater than 3 cm were found to have occult nodal disease at pathological dissection. There was an approximately 7.3-fold increase in the risk of occult nodal disease in patients with central tumors (adjusted OR: 7.3, 95% CI: 2.22-24.3). Nine of 24 patients (38%) with central tumors had occult nodal disease whereas only 10 of 120 patients (8.3%) with non-central tumors had occult nodal disease. Older age at resection demonstrated a slight but significant protective effect (adjusted OR: 0.95, 95% CI: 0.92-0.98).
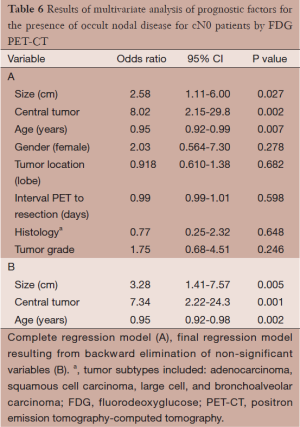
Full table
Discussion
Stage I NSCLC is located entirely within the lung parenchyma without regional, nodal, or distant involvement. Lobectomy with mediastinal lymphadenectomy is widely accepted as the standard approach for patients with stage I lung cancer (14). However, with the advancement of SBRT and its increasing use in lieu of resection for patients with early stage lung cancers, the evaluation of the performance of PET-CT (NPV) to detect nodal disease and the identification of predictors of the presence of clinically occult nodal disease are vital to the selection of optimal therapeutic approach for each patient.
According to the 7th edition AJCC/UICC TNM staging system, the overall 5-year survival rates of pathological stage IA and IB NSCLC are 73% and 58%, respectively. Within stage IA, 5-year survival rates decline from 77% for T1a to 71% for T1b (9,11). The association of increasing tumor size with shorter survival has been demonstrated by other investigators (15-19). In our series, increasing tumor size was associated with a significantly greater risk of having a final pathologic stage more advanced than IA/IB. Consistent with prior studies, we found a significantly higher risk of occult nodal metastasis as primary tumor size increases (8). Other investigators reported a high prevalence of occult N1 and N2 disease in clinical T1 lesions with the risk increasing 2-fold for tumors with diameter larger than 2 cm compared to those 1 cm or smaller (20-23). Our finding in multivariate analysis of a 1.8-fold increase in the risk of occult nodal disease for each higher T category in the 7th ed. of the AJCC staging system is similar to the relationship reported by Veeramachaneni et al. (19), who used final pathologic stage as an index of tumor size in their survival analysis, demonstrated significantly shorter survival at higher stages (larger tumor size).
Previous studies have reported the importance of anatomic lobectomy for NSCLC, including stage IA disease (14,24). However, recently there has been debate regarding the role of non-anatomical resection or SBRT for lung cancers smaller than 2 cm in diameter and without evidence of nodal or distant involvement on PET-CT. All patients with primary tumors smaller than 2 cm were found to be free of occult nodal disease in our analysis. Furthermore, previous retrospective studies have observed no difference in survival of patients who had non-anatomic resection of small lung cancers compared to those who underwent traditional surgical management (25,26). To our knowledge there have been no published prospective randomized trials specifically addressing this question in patients who were clinically staged as stage I by either the AJCC 6th or 7th ed. with PET-CT.
Prior studies have noted the improved performance of integrated PET-CT compared to CT or PET alone to evaluate both solitary pulmonary nodules and lymph node involvement for the staging of NSCLC (27-31). One objective of the present study was to add to previous reports of the NPV of FDG PET-CT for nodal disease in patients with stage I lung cancer. One analysis of 297 stage IA NSCLC (6th edition) patients and another of 197 patients reported NPVs for the detection of N1-N2 disease to be 85% and 87% respectively (15,19). For N2 disease specifically, the NPVs were 95% and 93% respectively. Our series, which demonstrates a NPV for clinically occult N1-N2 disease of 87% and a NPV for N2 of 92%, is consistent with these results and closely mirrors the NPVs reported earlier this year in the meta-analysis by Wang et al. (8). However, two additional series which looked solely at mediastinal (N2) lymph node involvement reported a higher rate of occult mediastinal disease: Gómez-Caro et al. and Al-Sarraf et al. documented NPVs of 85.6% (Gómez-Caro) and 84%, respectively (12,32). While there is some variability in these reports, our data and those in the recently published meta-analysis, suggest that PET-CT may be appreciably poorer at staging patients who appear to have larger primary tumors. The appearance of this trend both in our series and a meta-analysis of approximately 1,100 patients suggests that PET-CT alone may not be appropriate in the staging of patients with larger primary tumors (8). If the NPV of PET-CT for occult nodal metastases is truly 85-87%, then the identification and acceptance of predictors of nodal disease (tumor size, tumor location) to identify patients for whom noninvasive staging procedures and nonsurgical treatment options should be offered more cautiously could prove highly valuable to avoid under-treatment with radiotherapy.
Previous series have also shown centrally located tumors to be associated with a higher incidence of occult nodal disease (N2) than peripherally located tumors (7,12,33). In our analyses occult nodal disease (pN1-N2) was the dependent variable and the association between central tumor location and occult nodal disease was seen even in multivariate analyses controlling for primary lesion size (12). Other investigators have noted that the basis for this association may be a failure of FDG PET-CT to distinguish involved lymph nodes from the primary tumor’s activity due to an obscuring effect caused by the tumor’s proximity to the mediastinum (12).These analyses support the use of invasive mediastinal staging prior to resection of centrally located tumors.
One aspect of our study is the inconsistency of the reporting of nodule and nodal SUV uptake in our dataset. As a result, we could not include SUV uptake in our regression analysis. Previous studies have demonstrated that a SUVmax cutoff of 2.5 can be used to predict nodule malignancy and to a degree, 5-year survival (6,34). Furthermore, SUV and SUVmax have been predictive of intratumoral lymphatic vessel invasion and aggressiveness of T1 NSCLC nodules (35,36). The main limitation of this study is that the analysis was retrospective. To determine the true incidence of clinically occult nodal disease among patients with negative nodal uptake on PET-CT, prospective studies are warranted.
The results of this study demonstrate a satisfactory NPV for nodal involvement of NSCLC, especially for IA disease. However, overall underestimation of true pathological stage by integrated PET-CT is not uncommon, particularly with larger and centrally located tumors, risk factors that are generally acknowledged for occult nodal disease. When considering the utility of endoscopic or surgical mediastinal staging in such cases, the likelihood of occult nodal disease must be weighed against the false-negative rates for invasive staging procedures in clinically node-negative cases, which are generally greater than 10%. When contemplating nonsurgical treatment of early stage lung cancer, particularly SBRT, which affords no histological confirmation of nodal status, appreciation of the risk and consequences of occult nodal metastases is important, and should be made clear to patients considering nonsurgical treatment options, especially in operable patients. Invasive mediastinal staging should be considered for patients with larger or centrally located tumors with no evidence of nodal disease on PET-CT.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sandra Vermuelen, Kevin T. Murphy, Huan Giap) for the series “SBRT/SRS in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.06.06). The series “SBRT/SRS in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB). Informed consent was waived by the IRB as it was a retrospective chart review.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-20S.
- Scott WJ, Howington J, Feigenberg S, et al. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:234S-42S.
- Herder GJ, Kramer H, Hoekstra OS, et al. Traditional versus up-front [18F] fluorodeoxyglucose-positron emission tomography staging of non-small-cell lung cancer: a Dutch cooperative randomized study. J Clin Oncol 2006;24:1800-6. [PubMed]
- Pastorino U, Landoni C, Marchiano A, et al. Fluorodeoxyglucose uptake measured by positron emission tomography and standardized uptake value predicts long-term survival of CT screening detected lung cancer in heavy smokers. J Thorac Oncol 2009;4:1352-6. [PubMed]
- van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 2002;359:1388-93. [PubMed]
- Wang J, Welch K, Wang L, et al. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer 2012;13:81-9. [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [PubMed]
- Harpole DH Jr, Herndon JE 2nd, Young WG Jr, et al. Stage I nonsmall cell lung cancer. A multivariate analysis of treatment methods and patterns of recurrence. Cancer 1995;76:787-96. [PubMed]
- Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:593-602. [PubMed]
- Al-Sarraf N, Aziz R, Gately K, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg 2008;33:104-9. [PubMed]
- American Thoracic Society. Medical section of the American Lung Association. Clinical staging of primary lung cancer. Am Rev Respir Dis 1983;127:659-64. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Casiraghi M, Travaini LL, Maisonneuve P, et al. Lymph node involvement in T1 non-small-cell lung cancer: could glucose uptake and maximal diameter be predictive criteria? Eur J Cardiothorac Surg 2011;39:e38-43. [PubMed]
- Gajra A, Newman N, Gamble GP, et al. Impact of tumor size on survival in stage IA non-small cell lung cancer: a case for subdividing stage IA disease. Lung Cancer 2003;42:51-7. [PubMed]
- Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:694-705. [PubMed]
- Naruke T, Goya T, Tsuchiya R, et al. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg 1988;96:440-7. [PubMed]
- Veeramachaneni NK, Battafarano RJ, Meyers BF, et al. Risk factors for occult nodal metastasis in clinical T1N0 lung cancer: a negative impact on survival. Eur J Cardiothorac Surg 2008;33:466-9. [PubMed]
- Asamura H, Nakayama H, Kondo H, et al. Lymph node involvement, recurrence, and prognosis in resected small, peripheral, non-small-cell lung carcinomas: are these carcinomas candidates for video-assisted lobectomy? J Thorac Cardiovasc Surg 1996;111:1125-34. [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Prognostic factors in clinical stage I non-small cell lung cancer. Ann Thorac Surg 1999;67:927-32. [PubMed]
- Sugi K, Nawata K, Fujita N, et al. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg 1998;22:290-4; discussion 294-5. [PubMed]
- Flieder DB, Port JL, Korst RJ, et al. Tumor size is a determinant of stage distribution in t1 non-small cell lung cancer. Chest 2005;128:2304-8. [PubMed]
- Ishida T, Yano T, Maeda K, et al. Strategy for lymphadenectomy in lung cancer three centimeters or less in diameter. Ann Thorac Surg 1990;50:708-13. [PubMed]
- Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 2003;125:924-8. [PubMed]
- Shiraishi T, Shirakusa T, Iwasaki A, et al. Video-assisted thoracoscopic surgery (VATS) segmentectomy for small peripheral lung cancer tumors: intermediate results. Surg Endosc 2004;18:1657-62. [PubMed]
- Behzadi A, Ung Y, Lowe V, et al. The role of positron emission tomography in the management of non-small cell lung cancer. Can J Surg 2009;52:235-42. [PubMed]
- Bryant AS, Cerfolio RJ. The maximum standardized uptake values on integrated FDG-PET/CT is useful in differentiating benign from malignant pulmonary nodules. Ann Thorac Surg 2006;82:1016-20. [PubMed]
- Cerfolio RJ, Ojha B, Bryant AS, et al. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg 2004;78:1017-23; discussion 1017-23. [PubMed]
- Reed CE, Harpole DH, Posther KE, et al. Results of the American College of Surgeons Oncology Group Z0050 trial: the utility of positron emission tomography in staging potentially operable non-small cell lung cancer. J Thorac Cardiovasc Surg 2003;126:1943-51. [PubMed]
- Vansteenkiste JF, Stroobants SG, De Leyn PR, et al. Mediastinal lymph node staging with FDG-PET scan in patients with potentially operable non-small cell lung cancer: a prospective analysis of 50 cases. Leuven Lung Cancer Group. Chest 1997;112:1480-6. [PubMed]
- Gómez-Caro A, Garcia S, Reguart N, et al. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computer tomography scan. Eur J Cardiothorac Surg 2010;37:1168-74. [PubMed]
- Ketchedjian A, Daly BD, Fernando HC, et al. Location as an important predictor of lymph node involvement for pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2006;132:544-8. [PubMed]
- Divisi D, Di Tommaso S, Di Leonardo G, et al. 18-fluorine fluorodeoxyglucose positron emission tomography with computerized tomography versus computerized tomography alone for the management of solitary lung nodules with diameters inferior to 1.5 cm. Thorac Cardiovasc Surg 2010;58:422-6. [PubMed]
- Higashi K, Ueda Y, Yagishita M, et al. FDG PET measurement of the proliferative potential of non-small cell lung cancer. J Nucl Med 2000;41:85-92. [PubMed]
- Yoshioka M, Ichiguchi O. Selection of sublobar resection for c-stage IA non-small cell lung cancer based on a combination of structural imaging by CT and functional imaging by FDG PET. Ann Thorac Cardiovasc Surg 2009;15:82-8. [PubMed]


