
Risk factors of developing visceral metastases at diagnosis in prostate cancer patients
Introduction
Prostate cancer (PCa) is expected to account for 19% of all new tumor cases and 8% of all cancer death in male of United States (1-3). The most typical metastatic pattern including the lymph node (LN) and bone metastases have been studied comprehensively (4-6). However, little attention has been focused on the risk factors and prognosis of atypical metastases including liver, brain, and lung metastases in stage IV PCa patients. Previous studies (1,7-9) reported that visceral involvement in PCa might be correlated to worse survival outcomes. The visceral metastatic site and frequency of occurrence were described based on a large population using SEER database. Population-base estimates of the incidence and prognosis of visceral metastases at diagnosis of PCa are lacking. The risk factors of visceral metastases and the impact of these risk factors on the survival of patients with stage IV PCa were investigated in this study.
Methods
Data source
The SEER database includes information on cancer incidence, therapeutic management, and survival outcomes for approximately 30% of the US population. Clinical data on the absence or presence of visceral metastases including brain, liver, and lung at the time of PCa diagnosis was released in 2017 for patients diagnosed as PCa from 2010 to 2015. The incidence rates of PCa were calculated in this study. All rates were adjusted to the 2000 US standard population and expressed by 100,000 person-years. The annual percentage changes (APCs) of incidence of PCa were calculated based on the data from 1973 to 2015. The APCs of incidence of metastatic PCa were calculated based on the data from 2010 to 2015.
Patients
Patients with confirmed age and active follow-up who were diagnosed as having a primary, stage IV PCa in the SEER database from 2010 to 2015 were identified. The diagnosis of PCa was confirmed as adenocarcinoma (NOS, 8140/3) pathologically. Patients with carcinoma in situ were not included in this study. Patients for whom the absence or presence of visceral metastases at diagnosis was unknown were excluded. Subsequently, patients who were diagnosed at autopsy or via death certification, as well as patients who had an unknown follow up, grade, T stage, N stage, M stage, were also removed.
Statistical analysis
The demographics and clinical characteristics across visceral metastatic groups were compared using the Chi-square test. The relationships between PSA and visceral metastases were explored by constructing smoothing plots. A two-piecewise linear regression model was used to conduct the threshold effect of PSA on visceral metastases according to the smoothing plot. Multivariable logistic regression was used to determine the risk factors associated with the presence of visceral metastases at diagnosis. Multivariable Cox regression was performed to identified covariates associated with overall survival (OS) and cancer-specific survival (CSS) using the same variables as in the logistic regression model described herein. Survival estimates were obtained using the Kaplan-Meier method. The National Cancer Institute’s Joinpoint Regression program, version 4.5.0.1 was used to calculate APCs (10). Statistical analyses were performed using R software (version 3.4.4; R Foundation). Two-sided P<0.05 was considered to denote statistical significance.
Results
Incidence rate of bone and visceral metastatic PCa
PCa incidence rates increased 2.7% (95% CI, 1.9% to 3.5%, P<0.001) per year during 1973–1988. Rates increased significantly during 1988–1992; APC, 17.7% (95% CI, 10.9% to 25%, P<0.001), but did not increase −10.5% (95% CI, −20.2% to 0.4%, P=0.1) per year during 1995. The rate also did not increase during 1995–2000; APC, 2.6% (95% CI, −1.3% to 6.7%, P<0.2). Rates decreased significantly during 2009–2009 and 2009–2015; APC, −1.2% (95% CI, −2.4% to 0%, P<0.001) and −7.6% (95% CI, −9.5% to −5.7%, P<0.001), respectively (Figure 1A). The incidence rate of IV PCa, and metastatic PCa including bone metastases and visceral metastases were also increasing during the study period [2010–2015] (Figure 1B).
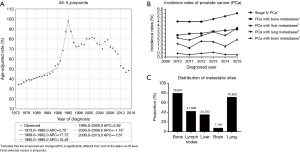
Baseline characteristics
Overall, 13,092 patients with stage IV PCa were identified. A total of 598 patients were with visceral metastatic PCa. The average age at diagnosis of visceral metastatic PCa was 68.296 years (median 68 years). The majority of visceral metastatic patients had lung metastases (70.903%). The majority of visceral metastatic patients (79.097%) accompanied with bone metastases. A total of 250 visceral metastatic patients (41.806%) accompanied with LN metastases. Overall, 73 visceral metastatic patients (11.873%) had two or three visceral metastatic sites involved at diagnosis. In addition, 473 (79.097%) patients were recorded as having bone plus visceral metastases. The characteristics of patients were stratified according to the different metastatic sites (Figure 1C, Table 1).
Table 1
| Singe site metastasis | None (%) | Brain metastasis only (%) | Liver metastasis only (%) | Lung metastasis only (%) | A least two metastases sites (%) | P value |
|---|---|---|---|---|---|---|
| N | 12,494 | 32 | 142 | 353 | 71 | |
| Age (years) | 66.145±9.693 | 68.156±9.392 | 68.063±9.513 | 68.470±10.648 | 67.958±10.678 | <0.001 |
| PSA value | 41.832±37.886 | 66.097±39.095 | 60.014±39.883 | 65.491±37.960 | 58.145±39.068 | <0.001 |
| Year | 2,012.755±1.720 | 2,012.625±1.792 | 2,012.641±1.694 | 2,012.992±1.703 | 2,012.380±1.633 | 0.023 |
| Grade | <0.001 | |||||
| Grade I | 116 (0.928) | 0 (0.000) | 1 (0.704) | 4 (1.133) | 0 (0.000) | |
| Grade II | 1,641 (13.134) | 6 (18.750) | 9 (6.338) | 25 (7.082) | 5 (7.042) | |
| Grade III | 10,671 (85.409) | 26 (81.250) | 128 (90.141) | 320 (90.652) | 65 (91.549) | |
| Grade IV | 66 (0.528) | 0 (0.000) | 4 (2.817) | 4 (1.133) | 1 (1.408) | |
| T stage | <0.001 | |||||
| T1 | 2,702 (21.626) | 16 (50.000) | 39 (27.465) | 124 (35.127) | 18 (25.352) | |
| T2 | 2,985 (23.891) | 11 (34.375) | 51 (35.915) | 122 (34.561) | 21 (29.577) | |
| T3 | 3,199 (25.604) | 2 (6.250) | 16 (11.268) | 49 (13.881) | 9 (12.676) | |
| T4 | 3,608 (28.878) | 3 (9.375) | 36 (25.352) | 58 (16.431) | 23 (32.394) | |
| N stage | <0.001 | |||||
| N0 | 6,309 (50.496) | 25 (78.125) | 79 (55.634) | 211 (59.773) | 33 (46.479) | |
| N1 | 6,185 (49.504) | 7 (21.875) | 63 (44.366) | 142 (40.227) | 38 (53.521) | |
| M stage | <0.001 | |||||
| M0 | 6,435 (51.505) | 0 (0.000) | 0 (0.000) | 0 (0.000) | 0 (0.000) | |
| M1 | 6,059 (48.495) | 32 (100.000) | 142 (100.000) | 353 (100.000) | 71 (100.000) | |
| Bone metastasis | <0.001 | |||||
| No | 7,069 (56.579) | 7 (21.875) | 30 (21.127) | 75 (21.246) | 13 (18.310) | |
| Yes | 5,425 (43.421) | 25 (78.125) | 112 (78.873) | 278 (78.754) | 58 (81.690) | |
| Surgery | <0.001 | |||||
| No | 6,825 (54.626) | 30 (93.750) | 114 (80.282) | 301 (85.269) | 60 (84.507) | |
| Yes | 5,669 (45.374) | 2 (6.250) | 28 (19.718) | 52 (14.731) | 11 (15.493) | |
PSA over 80 ng/mL was associated with a higher risk of developing visceral metastases at diagnosis
A nonlinear relationship between PSA and visceral metastases was identified. Overall, the risk of visceral metastases was increased with the elevated of PSA value (Figure 2A,B,C). When PSA value was divided into five groups including 4–20, 20–40, 40–60, 60–80, and >80 ng/mL, the risk of developing visceral metastases was varied accompanied with the elevated of PSA value (Figure 2A,B,C). It was obvious that the risk of visceral metastases was dramatically increased with the elevated of PSA value in patients with a PSA value >80 ng/mL (Figure 2C). The effect of PSA >80 ng/mL was then validated after adjusting for the confounders potentially associated with visceral metastases, including T stage, grade, bone metastasis, age, N stage, and surgery. In this study, PSA >80 ng/mL was an independent risk factor for predicting a higher risk of developing visceral metastases at diagnosis in patients with stage IV PCa (1.545, 95% CI, 1.233 to 1.935, P<0.001). Patients with PSA >80 ng/mL had 1.545-fold higher risk of developing visceral metastases compared with those with PSA <20 ng/mL (P<0.001). In T1 stage, patients with PSA >80 ng/mL had 2.754-fold higher risk of developing visceral metastases compared with those with PSA <20 ng/mL (P<0.001) (Table 2).
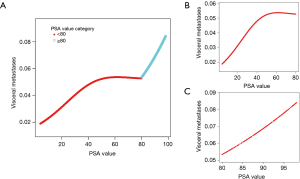
Table 2
| Exposure | Multivariate regression analysis |
|---|---|
| Grade | |
| Grade I | 1 |
| Grade II | 0.848 (0.326, 2.209), 0.73606 |
| Grade III | 1.022 (0.410, 2.550), 0.96290 |
| Grade IV | 2.374 (0.746, 7.557), 0.14334 |
| Bone metastasis | |
| No | 1 |
| Yes | 2.906 (2.266, 3.726), <0.00001 |
| Age | 1.005 (0.997, 1.013), 0.22947 |
| N stage | |
| N0 | 1 |
| N1 | 1.223 (1.019, 1.466), 0.03029 |
| Surgery | |
| No | 1 |
| Yes | 0.499 (0.384, 0.649), <0.00001 |
| PSA stratification | |
| <20 | 1 |
| ≥20, <40 | 1.051 (0.777, 1.422), 0.74710 |
| ≥40, <60 | 1.357 (0.961, 1.916), 0.08304 |
| ≥60, <80 | 1.131 (0.730, 1.754), 0.58085 |
| ≥80 | 1.545 (1.233, 1.935), 0.00016 |
Risk factors for visceral metastases at diagnosis
Multivariable logistic regression analysis among patients was used for exploring the risk factors of visceral metastases at diagnosis of PCa. Overall, bone metastasis, PSA >80 ng/mL and LN metastasis were significantly associated with greater odds of having visceral metastases at diagnosis. However, patients underwent surgery were associated with significantly smaller odds of having visceral metastases at diagnosis.
Survival analysis
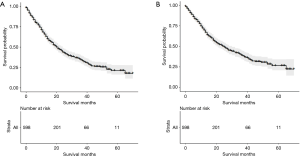
The median OS was 21 (95% CI, 18–24) months (Figure 3A) and the median CSS was 25 (95% CI, 21–30) months (Figure 3B). After stratifying visceral metastatic patients according to the metastatic sites, respective median OS and CSS were 16 and 16 months with brain metastasis, 16 and 17 months with liver metastasis, 32 and 37 months with lung metastasis, and 12 and 14 months with a least two metastases sites (P<0.01) (Figure 4A,B).
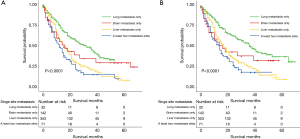
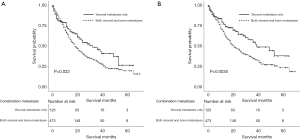
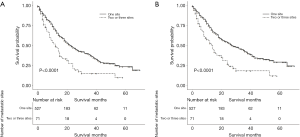
After stratifying visceral metastatic patients according to the number of metastatic sites involved, patients with one site involved had significantly longer OS and CSS than those with two or three metastatic sites involved (23 and 28 vs. 12 and 14, respectively) (Figure 5A,B). After stratifying visceral metastatic patients by visceral metastasis involved only and bone plus visceral metastasis involved, patients with metastasis involved only had significantly longer OS and CSS than those with bone plus visceral metastasis involved (32 and 39 vs. 18 and 21, respectively) (Figure 6A,B).
On multivariable Cox regression for OS and CSS among patients with visceral metastases at diagnosis, patients with bone metastasis, older age, LN metastases, bone plus visceral metastases, and two or three metastatic sites were associated with significantly greater risk of having shorter OS and CSS. Conversely, patients underwent surgery were associated with significantly smaller risk of having longer OS and CSS. However, T4 stage was associated with significantly greater risk of having shorter OS only. After adjusting for confounders, the number of the site of visceral metastases represented an independent predictor of OS. Particularly, patients with two or three metastatic sites had 1.604-fold higher risk of shorter OS compared with those with one metastatic site (P<0.05). And patients with bone plus visceral metastases had 1.410-fold higher risk of shorter OS compared with those with visceral metastasis only. Similarly, patients with two or three metastatic sites had 1.739-fold higher risk of shorter CSS compared with those with one metastatic site (P<0.05). And patients with bone plus visceral metastases had 1.611-fold higher risk of shorter CSS compared with those with visceral metastasis only (Table 3).
Table 3
| Exposure | Multivariate Cox regression analysis | ||||
|---|---|---|---|---|---|
| Overall survival | Cancer-specific survival | ||||
| Model 1 non-adjusted | Model 2 adjusted | Model 1 non-adjusted | Model 2 adjusted | ||
| Combination metastasis | |||||
| Visceral metastasis only | 1 | 1 | 1 | 1 | |
| Both visceral and bone metastases | 1.374 (1.019, 1.852), 0.03701 | 1.410 (1.039, 1.913), 0.02747 | 1.573 (1.122, 2.206), 0.00860 | 1.611 (1.141, 2.275), 0.00677 | |
| Number of metastatic sites | |||||
| One sites | 1 | 1 | 1 | 1 | |
| Two or three sites | 1.633 (1.205, 2.214), 0.00157 | 1.604 (1.180, 2.180), 0.00254 | 1.772 (1.284, 2.444), 0.00049 | 1.739 (1.258, 2.405), 0.00082 | |
| Age category | |||||
| <70 | 1 | 1 | 1 | 1 | |
| ≥70 | 1.608 (1.279, 2.022), 0.00005 | 1.621 (1.286, 2.043), 0.00004 | 1.407 (1.098, 1.803), 0.00699 | 1.429 (1.112, 1.836), 0.00524 | |
| N stage | |||||
| N0 | 1 | 1 | 1 | 1 | |
| N1 | 1.244 (0.975, 1.588), 0.07920 | 1.257 (0.977, 1.618), 0.07520 | 1.241 (0.954, 1.614), 0.10758 | 1.250 (0.953, 1.639), 0.10690 | |
| T stage | |||||
| T1 | 1 | 1 | 1 | 1 | |
| T2 | 1.032 (0.779, 1.366), 0.82644 | 1.023 (0.766, 1.364), 0.87891 | 1.002 (0.739, 1.358), 0.99224 | 0.992 (0.725, 1.357), 0.96164 | |
| T3 | 1.166 (0.803, 1.691), 0.41986 | 1.146 (0.785, 1.675), 0.48001 | 1.293 (0.877, 1.907), 0.19471 | 1.281 (0.862, 1.903), 0.22067 | |
| T4 | 1.481 (1.071, 2.047), 0.01748 | 1.476 (1.061, 2.053), 0.02066 | 1.399 (0.985, 1.987), 0.06107 | 1.392 (0.974, 1.990), 0.06967 | |
| Surgery | |||||
| No | 1 | 1 | 1 | 1 | |
| Yes | 0.718 (0.516, 1.000), 0.05015 | 0.709 (0.508, 0.989), 0.04277 | 0.730 (0.511, 1.043), 0.08398 | 0.715 (0.499, 1.024), 0.06688 | |
Discussion
In this study, the incidence of APC of identified PCa and stage IV PCa was identified. Overall, the incidence rate of PCa was decreased since 1992. However, the incidence rate of stage IV PCa was still increased annually. The APC of identified bone metastases, brain metastases, lung metastases, and liver metastases with newly diagnosed PCa was also identified. The subsequent survival of these patients was calculated. We found that the incidence proportion of lung metastases was highest among patients with visceral metastases. Median survival from diagnosis varied significantly by the different sites of visceral metastases. In addition, we report for the first time, to our knowledge, population-based risk factors associated with visceral metastatic stage IV PCa. The risk factors on survival outcome of visceral metastatic PCa were also investigated.
Visceral involvement was reported to be associated with worse clinical outcomes (11). So far, however, the risk factors on visceral metastases and the impact of visceral metastases on OS and CSS in patients with stage IV PCa has not been thoroughly elucidated (12,13). Although previous studies (1,4) mainly focused on the commonly spreads sites of PCa including the regional nodes and the skeleton, the risk of visceral metastases could not be negligible. Thus, we thought to explore the risk factors and evaluate the impact of the visceral metastatic sites on OS and CSS in a large cohort of patients with PCa.
To date, the characteristics of potential risk factors of developing visceral metastases in PCa have not been thoroughly elucidated. It is clearly that PSA was associated with the survival outcomes of PCa. However, the clinical effects of PSA in predicting the risk of metastasis have not been well established. Stattin et al. (14) reported that the PSA could predict the long-term risk of metastatic PCa. Risk stratification of distant metastasis was conducted by PSA in former study. Vertosick et al. (15) stated that risk stratification conducted by PSA was far greater than that reported for race or other demographic characteristics. To the best of our knowledge, it is the first time to evaluate that predictive effect of PSA on predicting the risk of developing visceral metastases in patients with stage IV PCa. In this study, PSA was stratified into five groups and PSA >80 ng/mL was found to be as an independent risk factor for predicting visceral metastases in patients with stage IV PCa. PSA >80 ng/mL had 1.545-fold higher risk of developing visceral metastases compared with those with PSA <20 ng/mL.
The OS and CSS of visceral metastases patients were conducted in this study. The OS and CSS varied based on the involvement sites of visceral metastases and the number of visceral metastasis involved. Guijarro et al. (16) found that patients with visceral involvement has a worse prognosis compared with exclusively LN metastasis, exclusively bone metastasis, or LN plus bone metastasis, which was in accordance with Mazzone’s (17) research. In addition, Mazzone et al. (17) also suggested that the prognosis of patients with bone involvement alone was inferior to those with LN involvement alone. Furthermore, Gandaglia et al. (11) showed that OS and CSS of patients with LN metastases superior to those with bone metastases, which was superior to those with visceral metastases. However, they did not investigate the impact of the different visceral metastatic sites on survival. To the best of our knowledge, it is the first time to evaluate the impact of the specific visceral metastatic sites on OS and CSS in visceral metastatic PCa patients. In this study, lung metastasis only had the best OS outcomes than liver metastasis only and brain metastasis only. The brain metastasis only had the worse OS outcomes than liver metastasis only and the lung metastasis only. In Guijarro’s study, they also showed that metastases number was not the prognostic factors of OS and progression-free survival (PFS) (16). In this study, visceral metastases with corresponding bone involvement conferred worse survival outcomes compared with visceral metastases alone, which was consistent with Mazzone’s (17) study. Moreover, we found that the number of metastases was significantly associated with survival, and presence of one metastatic site also results in longer OS and CSS than multiple metastatic sites involved in visceral metastatic patients. Whitney et al. (18) suggested that age is a prognostic factor of OS, but they did not define a threshold value. In this study, we found that age ≥70 had higher risk of shorter OS compared with those with age <70. However, Guijarro et al. (16) showed in their study that age was not significantly associated with OS and PFS in patients with metastatic prostatic cancer. Guijarro et al. (16) also found that clinical stage was not the prognostic factors of OS and PFS in patients with metastatic prostatic cancer, but this study indicated that T4 stage was associated with poor OS in patients with visceral metastases although it has no significant difference with CSS.
There are several clinical implications in this study. Firstly, the identification of the bone metastasis, the LN metastases, and PSA value ≥80 ng/mL as risk factors may be important to predict the visceral metastases. Secondly, the identification of the visceral plus bone metastases, the number of visceral sites involved, age over 70 years as prognostic factors might be critical to predict the OS and CSS in patients with visceral metastases. However, T4 stage was only associated with poor OS in patients with visceral metastases. Hence, patients with prognostic factors mentioned above might underwent more aggressive approaches rather than conventional treatment modalities (11).
There were some limitations in this study. Firstly, we were able to describe only the absence or presence of visceral metastases at initial diagnosis. Further information concern disease recurrence or subsequent sites of disease involvement were not provided in the SEER database. Patients who subsequently developed visceral metastases would not be calculated in this study. Secondly, the comorbidities, performance status, smoking status, hypertension status, and diabetes status were not available in the SEER database. Thirdly, patients’ therapeutic strategies for visceral metastases were not recorded in the SEER database. T4 stage was only associated with poor OS in patients with visceral metastases. Therefore, we assumed that those patients might undergo more aggressive approaches rather than conventional treatment strategies. However, due to the inherent defect of the SEER database, the specific therapeutic strategies of patients cannot be accessed. Hence, this limitation could only be discussed in further study.
Conclusions
The incidence rate was increased among patients with visceral metastases in stage IV PCa at diagnosis. PSA over 80 ng/mL, the presence of bone metastasis and the presence of LN metastases were risk factors closely correlated with the development of visceral metastases in stage IV PCa patients. The presence of visceral plus bone metastases, two or three sites, age over 70 and T4 stage represent prognostic factors on survival outcomes in visceral metastatic PCa patients.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.05.31). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Since all information from the SEER database has been de-identified and no identifying information was used in this analysis, informed consent is not required for use of SEER data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shou J, Zhang Q, Wang S, et al. The prognosis of different distant metastases pattern in prostate cancer: A population based retrospective study. Prostate 2018;78:491-7. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 2014;74:210-6. [Crossref] [PubMed]
- Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999-2006. Prostate Cancer Prostatic Dis 2011;14:177-83. [Crossref] [PubMed]
- Briganti A, Passoni NM, Abdollah F, et al. Treatment of lymph node-positive prostate cancer: teaching old dogmas new tricks. Eur Urol 2014;65:26-7; discussion 28. [Crossref] [PubMed]
- Conteduca V, Caffo O, Fratino L, et al. Impact of visceral metastases on outcome to abiraterone after docetaxel in castration-resistant prostate cancer patients. Future Oncol 2015;11:2881-91. [Crossref] [PubMed]
- Mazzone E, Preisser F, Nazzani S, et al. Location of Metastases in Contemporary Prostate Cancer Patients Affects Cancer-Specific Mortality. Clin Genitourin Cancer 2018;16:376-384.e1. [Crossref] [PubMed]
- Leonel Almeida P, Jorge Pereira B. Local Treatment of Metastatic Prostate Cancer: What is the Evidence So Far? Prostate Cancer 2018;2018:2654572. [Crossref] [PubMed]
- Saad AM, Turk T, Al-Husseini MJ, et al. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 2018;18:688. [Crossref] [PubMed]
- Gandaglia G, Karakiewicz PI, Briganti A, et al. Impact of the Site of Metastases on Survival in Patients with Metastatic Prostate Cancer. Eur Urol 2015;68:325-34. [Crossref] [PubMed]
- Pond GR, Sonpavde G, de Wit R, et al. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol 2014;65:3-6. [Crossref] [PubMed]
- Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol 2012;199:367-72. [Crossref] [PubMed]
- Stattin P, Vickers AJ, Sjoberg DD, et al. Improving the Specificity of Screening for Lethal Prostate Cancer Using Prostate-specific Antigen and a Panel of Kallikrein Markers: A Nested Case-Control Study. Eur Urol 2015;68:207-13. [Crossref] [PubMed]
- Vertosick EA, Poon BY, Vickers AJ. Relative value of race, family history and prostate specific antigen as indications for early initiation of prostate cancer screening. J Urol 2014;192:724-8. [Crossref] [PubMed]
- Guijarro A, Hernandez V, de la Morena JM, et al. Influence of the location and number of metastases in the survival of metastatic prostatic cancer patients. Actas Urol Esp 2017;41:226-33. [Crossref] [PubMed]
- Mazzone E, Preisser F, Nazzani S, et al. Location of Metastases in Contemporary Prostate Cancer Patients Affects Cancer-Specific Mortality. Clin Genitourin Cancer 2018;16:376-84.e1. [Crossref] [PubMed]
- Whitney CA, Howard LE, Posadas EM, et al. In Men with Castration-Resistant Prostate Cancer, Visceral Metastases Predict Shorter Overall Survival: What Predicts Visceral Metastases? Results from the SEARCH Database. Eur Urol Focus 2017;3:480-6. [Crossref] [PubMed]


