
Gamma knife pallidotomy for treatment of Parkinson’s disease: long term results, clinical study
Introduction
The so-called “Leksell’s posteroventral pallidotomy” was reintroduced by Laitinen in 1992 and subsequently became extremely popular for treatment of tremor, bradykinesia, rigidity and L-DOPA induced dyskinesia in patients with Parkinson’s disease (PD) (1-4). The procedure consisted of making a radiofrequency lesion (RFP) in the internal segment of the globus pallidus (GPi). Multiple publications throughout the 1990s and the early 2000s attested to the safety and effectiveness of this procedure but the adaptation of deep brain stimulation (DBS) to the treatment of tremor and then PD, led to the near abandonment of pallidotomy (5). Interestingly some strong proponents of DBS have recently suggested that lesioning procedures including pallidotomy may be preferable to DBS in certain patients (6). At least one comparison, for instance, suggests that similar improvements in Parkinsonian symptoms can be achieved with radiofrequency Pallidotomy as with DBS in the pallidum (7). Rand published what is, to our knowledge, the first application of the Leksell gamma knife (LGK) to the performance of pallidotomy (8) and we and others subsequently published our experience with this technique (9-12). Only reports of rather limited numbers of patients with limited follow up periods have been published to this date and there are conflicting reports as to the safety and efficacy of gamma knife pallidotomy (GKP). In this report we describe our experience with 51 GKP procedures performed in 40 patients between 1993 and 2009. We believe that it is important to document this experience for the benefit of potential patients who may not be candidates for DBS or radiofrequency Pallidotomy but who may benefit from GKP.
Materials and methods
Between August 1993 and July 2001, 40 patients with advanced PD underwent a total of 51 GKP procedures (29 unilateral and 11 bilateral). The senior author (RFY) served as the neurosurgeon for all of the procedures with various radiation oncology and radiation physics colleagues and they represent all of the GKP procedures performed at our institution during the described time interval. In past reports we have included larger numbers of patients pooled from more than one institution but this report describes our experience at a single institution in which we have employed consistent selection criteria, surgical technique and follow up procedures. The mean follow up period for patients who underwent unilateral GK GKP was 91 months (range, 48-127 months) and for those who underwent bilateral GKP the mean follow up period following the second procedure was 74 months (range, 42-93 months).
Statistical analysis of data was performed using pairwise comparisons of the preoperative Unified Parkinson’s disease rating scale (UPDRS) scores and the scores at last follow up using the Wilcoxon signed rank test for each variable. For bilateral procedures comparisons were made of the scores prior to the first and second procedures and at last follow-up. Percentage changes from the mean preoperative values were calculated. Mean total daily Levodopa-equivalent doses were compared at baseline preoperatively and at last follow up using Student’s t-test.
Evaluation of PD
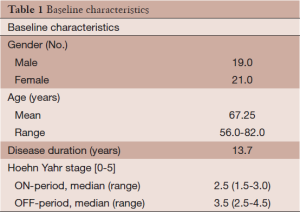
All patients had documented responses to L-DOPA preoperatively and many patients experienced severe disabling dyskinesias as a complication of their L-DOPA therapy. Demographics of the patient population are shown in Table 1. Twenty four patients exhibited absolute or relative contraindications to an open stereotactic procedure including chronic use of anticoagulants (13), severe cardiopulmonary problems (7), immune deficiency disorders (2), and bleeding diatheses (1). One patient had multiple cerebral cavernous malformations and it was thought impossible to find a safe trajectory for an open stereotactic procedure. For those patients who did not exhibit contraindications both RFP and GKP were offered and in more recent years DBS was also offered. Exclusion criteria included Parkinson’s Plus Syndromes or significant dementia.
Full table
Preoperatively all patients underwent videotaping in the defined “OFF” and “ON” medication states and subsequently blinded examiners determined UPDRS scores. Scoring was performed by a trained team of physical therapists, nurses and a PhD specialist in movement disorders. None of the authors of this report performed either the pre or postoperative evaluations. Pre and postoperative evaluations were part of a comprehensive prospective movement disorders program which included Gamma Knife lesioning (both thalamotomy and pallidotomy), radiofrequency lesioning, and DBS. This evaluation technique has been reported in detail in previous publications (12).
Follow up protocol
Scoring was repeated at 6 and 12 months postoperatively and yearly thereafter. MRI scans were obtained at the same intervals except that when follow-up reached 5 years MRI scanning was reduced to 2 or 3 years intervals. Eventually 26 patients died of causes unrelated to the GKP procedures and an additional four patients were lost to follow up for a variety of reasons.
Surgical technique
Our surgical technique for GKP has been described in considerable detail in several previous publications (11-13). Briefly, the Leksell Model G stereotactic frame was attached to the patient’s head utilizing a combination of mild intravenous sedation and local anesthetic infiltration at the pin sites. Every effort was made to minimize pitch, roll and yaw errors in frame placement. A non-contrast MRI scan was then obtained. The parameters for our MRI scan technique have also been recently published (13). The anterior and posterior commissures were identified and the inter-commissural distance was calculated using axial, coronal and sagittal images. A coronal scan was then examined 2-3 mm anterior to the mid-commissural point. A single isocenter was then positioned in the internal segment of the directly visualized wedge shaped internal segment of the GPi superior to the optic tract and medial to the internal capsule (12,13). An in house algorithm was used to correct for errors of pitch, roll and yaw in frame placement to determine the final X, Y and Z stereotactic target coordinates. A radiosurgical dose maximum of 140 Gy (output factor 0.80/0.87) was then administered using the Model U or Model C Leksell Gamma Unit (Gamma Knife) and the 4 mm secondary collimator. Subsequent to administration of the dose, the stereotactic frame was removed and the patient was observed overnight in the hospital and then discharged home the next morning. Currently all such procedures are performed on an outpatient basis.
Results
Imaging studies
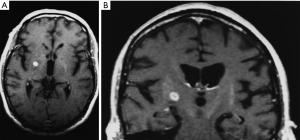
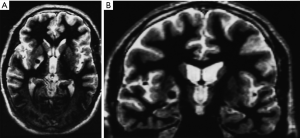
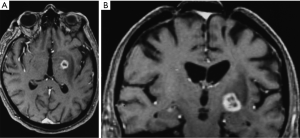
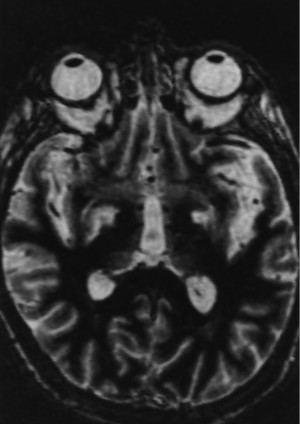
By 6 months after the procedures, our first follow-up interval, the lesions appeared as sharply circumscribed spherical regions on T1 weighted MRI images which enhanced intensely following administration of intravenous contrast material (Figure 1A,B). T2 weighted images sometimes showed a perilesional abnormality 6-12 months after the procedures but these imaging changes were not associated with any clinical symptoms (Figure 2A,B). Mean lesion volume as calculated to the outer edge of the zone of contrast enhancement on T1 images obtained one year after the procedures was 167.4 mm3. Two patients who developed complications of the treatments showed lesions considerably larger than expected, with lesion volumes of 1,436 and 904 mm3 (Figure 3A,B). Scans obtained many years after the procedures showed sharply demarcated lesions which enhanced minimally, if at all, following intravenous contrast administration.
Unilateral procedures
Twenty-nine patients underwent unilateral procedures only. There were no significant differences in the mean total levodopa-equivalent doses between the baseline preoperative amounts and the doses at the last follow up. The outcomes are summarized in Table 2. In the OFF-period overall UPDRS scores were improved by 18.4% at the last follow up period (71.4±17.1 baseline to 58.2±14.7), with overall motor scores improving 18.2% (52.7±15.9 baseline to 34.5±13.0). Contralateral bradykinesia improved 23.4% (11.1±3.4 baseline to 8.5±3.6), tremor 72.4% (2.9±0.7 baseline to 0.8±1.1) and rigidity 51.2% (4.1±2.5 baseline to 2.0±1.6). In the ON-period, dyskinesias improved 70% (2.8±1.1 baseline to 0.84±0.9) contralateral to the lesion and 16.7% (1.8±0.9 to 1.5±0.6) ipsilaterally. All of the improvements were statistically significant (P<0.05 or better) except for Activities of Daily Living (ADL) (P=0.26). All measures showed greater improvement in the first year than were sustained at the last evaluation.
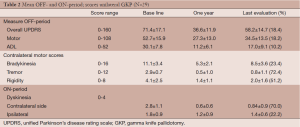
Full table
Bilateral procedures
Eleven patients underwent bilateral GKPs with a mean interval between the two procedures of 18.4 (range, 13-35) months (Figure 4). There were no significant differences in the mean total levodopa-equivalent scores between the baseline doses prior to the second procedure and at the last follow up. The outcomes are shown in Table 3. We have provided the baseline values for various outcomes parameters before the first procedure and then again immediately before the second procedure to allow comparisons of the effects of the first and second procedures separately and together. In summary the second procedure produced an incremental improvement in outcomes in the OFF medication state comparable to, although not quite as robust as, after the first procedure. For instance the overall UPDRS for this group was improved by 19.8% after the first procedure and by an additional 12.1% after the second procedure for an overall improvement of 31.9% combined for the bilateral procedures. Similar results pertain for motor scores and activities of daily living scores. Interestingly, ADL scores which showed a statistically insignificant improvement after unilateral GKP (10.2%) showed a further improvement after bilateral GKP of 21% which was statistically significant when compared to the baseline ADL scores before the first procedure. ON medication dyskinesia scores contralateral to the first lesion, which had improved by 72.4% after the first procedure, declined very slightly but remained improved by 69.1% after the second procedure. Dyskinesia scores ipsilateral to the first procedure had actually deteriorated by 42% between the first and second procedures presumably due to disease progression but they showed a 65% improvement after the second procedure. Thus there were very significant improvements bilaterally in dyskinesias after the bilateral procedures.

Full table
Complications
Two patients suffered contralateral complete homonymous hemianopsias following unilateral GKP. One of these patients also suffered contralateral mild hemiparesis. In both cases the complications were due to lesions, which developed larger than expected (Figure 3A,B). The visual field defects were permanent in both cases although the hemiparesis improved significantly. Interestingly none of the patients who underwent bilateral procedures suffered any complications. Thus the complication rate was 5.0% calculated on a per patient basis and 3.9% calculated on a per lesion basis.
Discussion
Surgical treatments for PD
It appears that DBS is the most popular current treatment for PD whether the target for stimulation is in the STN or GPi. The efficacy and safety of these two targets appears relatively equal for the treatment of the primary motor endpoints, although quality of life may be better with unilateral GPi vs. STN (14-17). Ablative procedures appear to have fallen into disuse although in the 1990s RFP was extremely popular and even more recently some authors who are strong proponents of DBS believe that there are some patients who are better suited for ablative procedures than for DBS (5,6). We would agree with this general sentiment, arguing however that in some cases LGK procedures might be even more suitable than RF procedures.
Results of GKP
We believe that this report has several strengths over prior reports of the results of RFP which include the fact that the evaluations were part of a prospective, blinded protocol and included all such procedures performed at a single institution. Unilateral RFP produced sustained and statistically significant improvements in multiple parameters of PD as measured by UPDRS scores. Most significant were improvements in dyskinesias and tremor, 70% and 72.4% improvements respectively and also in rigidity and bradykinesia. Lesser improvements were seen in overall UPDRS scores, and overall motor scores. Although there was a slight improvement in ADL scores these improvements were not statistically significant.
Bilateral GKP produced additional incremental improvements in more global representations of PD disabilities. Overall UPDRS scores which had improved by 19.8% after the first procedure increased to a 31.9% improvement after the second procedure. Overall motor scores were better by 20.2% after the first procedure and by 33.1% after the second procedure. Finally, ADL scores that improved only by 10.2% and were statistically insignificant after the first procedure, improved by 21.0% after the second procedure and this improvement was statistically significant. We believe this to be an important observation as improvements in ADL are particularly important for patients with PD from a functional standpoint. As with a unilateral procedure the second of a bilateral procedure produced a very significant improvement (65%) in contralateral dyskinesias. Thus the second of a bilateral procedure resulted in very important additional improvements in the abilities of patients with PD to function more normally.
Complications of GKP
We experienced two complications in our patient population, both with permanent homonymous hemianopsias and one also with hemiparesis that improved significantly over time. This represents a rate of complications of 4.2% considered on a per lesion basis and 5.4% considered on a per patient basis. These rates are comparable to the complication rates that we and others have published in the past for lesioning procedures with the LGK (2,5). For instance in our series of 203 Gamma Knife Thalamotomy procedures used to treat Essential Tremor we found a permanent complication rate of 3.9% (18). We believe that the current report combined with our recent previous report which when combined document a total of 250 GK lesioning procedures to treat movement disorders, demonstrate conclusively that the permanent complication rate of these procedures is in the range of 4% to 5% and confirms the relative safety of these procedures. Prior opinions that LGK lesioning procedures to treat movement disorders are dangerous or should not be performed, we believe, can no longer be justified (9,10,19). Interestingly none of the 11 patients in the current report who underwent bilateral GKP experienced complications of the procedures.
Comparison of GKP, RFP and DBS
GKP provides significant and durable improvements in motor performance in patients with PD. The magnitude of improvement is comparable to that which results from RFP Alkhani and Lozano (1) published an excellent review of the RFP literature but Fine, et al. provided perhaps the best long-term study of the results of unilateral RFP in 20 of a cohort of 40 patients (20). They demonstrated a 37% short term and an 18% long term improvement in overall OFF-period UPDRS scores and as with our results and other prior reports for GKP, noted deterioration in these results over time (20-24). Likewise, they demonstrated an 18% long term improvement in OFF-period total motor scores. Both of these results are virtually identical to our results with GKP. In addition they demonstrated a 70% improvement in ON-period dyskinesia scores again very similar to our own results and like us they noted that these results did not deteriorate over time. It should also be noted that the Fine, et al. report includes only 20 of 40 RFP procedures performed at their institution and the authors indicate that the 20 excluded patients probably represent those with worse outcomes and thus the Fine, et al. report represents the best case scenario for the outcomes of RFP. In the present report only a single patient lost to follow up less than one year after the procedures were performed is excluded from the follow up data.
Our results with bilateral GKP are also similar to the published results of bilateral RFP in that the second procedure provided an incremental improvement in all outcome measures although not quite as robust as after the first procedure. Parkin, et al. reported a 27% improvement in UPDRS motor scores after unilateral RFP and an additional improvement to 31% after the second procedure (22). They also reported an overall 40% abolition of dyskinesias after unilateral RFP, which increased to 63% after the second procedure. De Bie, et al. reported a 28% improvement in ADL scores after unilateral RFP and a further improvement to 33.3% after the second procedure (25). They also reported a 33.3% improvement in motor scores after the first procedure and a further improvement to 45.9% after the second procedure. These results are quite similar to ours although making comparisons of specific UPDRS scores is difficult since the reports described above for RFP represent very short term follow up periods and different assessment methods than in our report. In general however, the results of both RFP and GKP seem to indicate that the second of a bilateral procedure produces incremental additional improvements in motor function. The relative cost difference between a unilateral and bilateral procedures maybe an issue (26). The main difference in the outcomes after bilateral GKP in our experience and the published results for bilateral RFP is in the area of complications. For instance, De Bie, et al. described complications in 8/13 patients who underwent bilateral RFP including five with speech problems and one who was hemiplegic due to a delayed infarction whereas none of the 11 patients in our series who underwent bilateral GKP experienced any complications of the second procedure (25).
A recent series and another review suggests, and we tend to agree, that DBS is superior to pallidotomy over long term follow-up (7,27-29). However, evidence for the neuropsychiatric and cognitive decline complications of DBS has been mounting, and may suggest that further screening may be needed to identify appropriate candidates for DBS (30-32). Hardware related complications such as lead fractures and infections with DBS also need to be considered in the decision process as some studies suggest an incidence of 15-25% (29,33). The impacts of future need for diagnostic MR imaging (with a body coil) or surgery with electrocautery and the attendant risks also need to be considered in addition to the expected costs of battery replacement and programming for patients with implanted DBS systems. Finally there remains some controversy as to whether the STN or the GPi is the superior target for DBS to treat PD (14).
Although RFP has been largely abandoned in favor of DBS, particularly in the subthalamic region, there are certain patients who may not be candidates for DBS or for whom a lesioning procedure may offer a better risk to benefit ratio (5,6,34). In this regard we feel it is important to document not only the effectiveness of radiosurgical procedures for the treatment of movement disorders but the risk of complications based on long term observations of significant numbers of patients. This is particularly important because some other reports with limited number of patients or with no denominator to calculate the actual risk have suggested that radiosurgical procedures for the treatment of movement disorders are dangerous or should not be performed (19,35,36). Our experience as described in this and several other reports documents the small but significant risk of such radiosurgical procedures but we suggest that in comparison to both RPF and DBS, GKP deserves to be considered as a viable option, particularly in patients who may not be suitable candidates for any open stereotactic procedure. In this regard, it is interesting that in the excellent long term follow up report of Fine, et al. on RFP for PD there is no mention whatsoever of complications (20), yet other reports describe significant neurological complications of RFP including intracerebral hemorrhage often requiring craniotomy for treatment, neurological deficits without intracerebral hemorrhage and postoperative confusion (32,33,37-39). These later authors (35) concluded that: “Complications from stereotactic pallidotomy were not frequent. However, the residual symptoms from complications can be serious in many cases”.
Intriguingly, and as an area for possible future research, it has been suggested by one author that radiosurgery may include neuromodulatory changes separate from physical lesioning (40).
Conclusions
GKP is a safe and effective surgical treatment for PD. It should not be surprising that GKP and RFP produce similar improvements in UPDRS scores as they are merely two different methods of creating lesions within the GPi. Based on our long term experience GKP is certainly as safe as and perhaps safer than RFP. GKP is not as effective as DBS over long term follow up but because GKP can be performed in patients not suitable for DBS, is less invasive than RFP and avoids the problems and expense associated with DBS we believe that in certain selected patients GKP remains a useful procedure in the armamentarium of neurosurgeons that treat PD.
Acknowledgments
We gratefully acknowledge the assistance of our past radiation oncology and radiation physics colleagues in the performance of these procedures. We also thank our former colleague Ann Shumway-Cook, PhD who developed our scoring procedures and trained the physical therapy staff in the performance of the UPDRS scoring test procedures. We also thank Beverly Clark and Kim Jarvis RNs, Wanda McKinney and Emily Anderson for assistance with patient management and finally we thank Sachielle Zamprioli for assistance with data collection and statistical analysis.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “SBRT/SRS in Radiation Research”. The article has undergone external peer review.
Conflicts of Interest: Francisco Li is a paid consultant for Elekta AB, Sweden. The other authors declare no conflict of interest.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study is approved by the Institutional Review Board (IRB). This was a retrospective chart review and no patient signatures were required for the review. However, all patients signed an IRB informed consent for treatment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alkhani A, Lozano AM. Pallidotomy for parkinson disease: a review of contemporary literature. J Neurosurg 2001;94:43-9. [PubMed]
- Hariz MI, Bergenheim AT. A 10-year follow-up review of patients who underwent Leksell’s posteroventral pallidotomy for Parkinson disease. J Neurosurg 2001;94:552-8. [PubMed]
- Laitinen LV, Bergenheim AT, Hariz MI. Leksell’s posteroventral pallidotomy in the treatment of Parkinson’s disease. J Neurosurg 1992;76:53-61. [PubMed]
- Laitinen LV. Leksell’s unpublished pallidotomies of 1958-1962. Stereotact Funct Neurosurg 2000;74:1-10. [PubMed]
- Gross RE. What happened to posteroventral pallidotomy for Parkinson’s disease and dystonia? Neurotherapeutics 2008;5:281-93. [PubMed]
- Okun MS, Vitek JL. Lesion therapy for Parkinson’s disease and other movement disorders: update and controversies. Mov Disord 2004;19:375-89. [PubMed]
- Blomstedt P, Hariz GM, Hariz MI. Pallidotomy versus pallidal stimulation. Parkinsonism Relat Disord 2006;12:296-301. [PubMed]
- Rand RW, Jacques DB, Melbye RW, et al. Gamma Knife thalamotomy and pallidotomy in patients with movement disorders: preliminary results. Stereotact Funct Neurosurg 1993;61:65-92. [PubMed]
- Duma CM. Movement disorder radiosurgery--planning, physics and complication avoidance. Prog Neurol Surg 2007;20:249-66. [PubMed]
- Friedman DP, Goldman HW, Flanders AE, et al. Stereotactic radiosurgical pallidotomy and thalamotomy with the gamma knife: MR imaging findings with clinical correlation--preliminary experience. Radiology 1999;212:143-50. [PubMed]
- Young RF. Functional neurosurgery with the Leksell Gamma knife. Stereotact Funct Neurosurg 1996;66:19-23. [PubMed]
- Young RF, Shumway-Cook A, Vermeulen SS, et al. Gamma knife radiosurgery as a lesioning technique in movement disorder surgery. J Neurosurg 1998;89:183-93. [PubMed]
- Macdonald RL. eds. Neurosurgical Operative Atlas. New York: Thieme, 2008.
- Anderson VC, Burchiel KJ, Hogarth P, et al. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol 2005;62:554-60. [PubMed]
- Goodman RR, Kim B, McClelland S 3rd, et al. Operative techniques and morbidity with subthalamic nucleus deep brain stimulation in 100 consecutive patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry 2006;77:12-7. [PubMed]
- Okun MS, Fernandez HH, Wu SS, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol 2009;65:586-95. [PubMed]
- Zahodne LB, Okun MS, Foote KD, et al. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. J Neurol 2009;256:1321-9. [PubMed]
- Young RF, Li F, Vermeulen S, et al. Gamma Knife thalamotomy for treatment of essential tremor: long-term results. J Neurosurg 2010;112:1311-7. [PubMed]
- Okun MS, Stover NP, Subramanian T, et al. Complications of gamma knife surgery for Parkinson disease. Arch Neurol 2001;58:1995-2002. [PubMed]
- Fine J, Duff J, Chen R, et al. Long-term follow-up of unilateral pallidotomy in advanced Parkinson’s disease. N Engl J Med 2000;342:1708-14. [PubMed]
- de Bie RM, Schuurman PR, Bosch DA, et al. Outcome of unilateral pallidotomy in advanced Parkinson’s disease: cohort study of 32 patients. J Neurol Neurosurg Psychiatry 2001;71:375-82. [PubMed]
- Parkin SG, Gregory RP, Scott R, et al. Unilateral and bilateral pallidotomy for idiopathic Parkinson’s disease: a case series of 115 patients. Mov Disord 2002;17:682-92. [PubMed]
- Strutt AM, Lai EC, Jankovic J, et al. Five-year follow-up of unilateral posteroventral pallidotomy in Parkinson’s disease. Surg Neurol 2009;71:551-8. [PubMed]
- Valldeoriola F, Martínez-Rodríguez J, Tolosa E, et al. Four year follow-up study after unilateral pallidotomy in advanced Parkinson’s disease. J Neurol 2002;249:1671-7. [PubMed]
- De Bie RM, Schuurman PR, Esselink RA, et al. Bilateral pallidotomy in Parkinson’s disease: a retrospective study. Mov Disord 2002;17:533-8. [PubMed]
- Green AL, Joint C, Sethi H, et al. Cost analysis of unilateral and bilateral pallidotomy for Parkinson’s disease. J Clin Neurosci 2004;11:829-34. [PubMed]
- Esselink RA, de Bie RM, de Haan RJ, et al. Unilateral pallidotomy versus bilateral subthalamic nucleus stimulation in PD: a randomized trial. Neurology 2004;62:201-7. [PubMed]
- Esselink RA, de Bie RM, de Haan RJ, et al. Unilateral pallidotomy versus bilateral subthalamic nucleus stimulation in Parkinson’s disease: one year follow-up of a randomised observer-blind multi centre trial. Acta Neurochir (Wien) 2006;148:1247-55; discussion 1255. [PubMed]
- Seijo FJ, Alvarez-Vega MA, Gutierrez JC, et al. Complications in subthalamic nucleus stimulation surgery for treatment of Parkinson’s disease. Review of 272 procedures. Acta Neurochir (Wien) 2007;149:867-75; discussion 876. [PubMed]
- Appleby BS, Duggan PS, Regenberg A, et al. Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: A meta-analysis of ten years’ experience. Mov Disord 2007;22:1722-8. [PubMed]
- Smeding HM, Esselink RA, Schmand B, et al. Unilateral pallidotomy versus bilateral subthalamic nucleus stimulation in PD--a comparison of neuropsychological effects. J Neurol 2005;252:176-82. [PubMed]
- York MK, Lai EC, Jankovic J, et al. Short and long-term motor and cognitive outcome of staged bilateral pallidotomy: a retrospective analysis. Acta Neurochir (Wien) 2007;149:857-66; discussion 866. [PubMed]
- Blomstedt P, Hariz MI. Are complications less common in deep brain stimulation than in ablative procedures for movement disorders? Stereotact Funct Neurosurg 2006;84:72-81. [PubMed]
- Frighetto L, Bizzi J, Annes RD, et al. Stereotactic radiosurgery for movement disorders. Surg Neurol Int 2012;3:S10-6. [PubMed]
- Bonnen JG, Iacono RP, Lulu B, et al. Gamma knife pallidotomy: case report. Acta Neurochir (Wien) 1997;139:442-5. [PubMed]
- Friedman JH, Fernandez HH, Sikirica M, et al. Stroke induced by gamma knife pallidotomy: autopsy result. Neurology 2002;58:1695-7. [PubMed]
- de Bie RM, de Haan RJ, Schuurman PR, et al. Morbidity and mortality following pallidotomy in Parkinson’s disease: a systematic review. Neurology 2002;58:1008-12. [PubMed]
- Higuchi Y, Iacono RP. Surgical complications in patients with Parkinson’s disease after posteroventral pallidotomy. Neurosurgery 2003;52:558-71; discussion 568-71. [PubMed]
- Hua Z, Guodong G, Qinchuan L, et al. Analysis of complications of radiofrequency pallidotomy. Neurosurgery 2003;52:89-99; discussion 99-101. [PubMed]
- Régis J, Carron R, Park M. Is radiosurgery a neuromodulation therapy?: A 2009 Fabrikant award lecture. J Neurooncol 2010;98:155-62. [PubMed]


