Expression of HER-2 in surgical specimen and biopsy as a biomarker of metastasis in patients with osteosarcoma: a meta-analysis
Introduction
Osteosarcoma (OS) is the most common malignancy in bones and is limited to the metaphysis of long bones (1,2). In adolescents and young adults, OS is one of the most common primary malignant bone tumors. The survival rate of patients with OS increased with the introduction of advanced surgery and combination chemotherapy. However, a large proportion of patients are still involved in fatal metastasis, which significantly reduces the survival rate at present. Because the mechanism of oncogenes has not yet been fully elucidated, the ability to predict the metastasis of OS is currently very limited. Therefore, it’s urgently needed to understand the prognostic biomarkers of metastatic OS. These markers can identify patients at higher risk, so the patients can use more intensive and aggressive treatment at the time of initial diagnosis. So far, human epidermal growth factor receptor 2 (HER-2) expression has been identified as one of the potential prognostic biomarkers of OS (3).
Over-expression of HER-2/neu/C-erbB2/ErbB-2 is closely related to the degree of malignancy of many epithelial cell cancers. HER-2 high-expression tumors show strong migratory and invasive properties, poor sensitivity of chemotherapeutic drugs, poor post-hepatology, and recurrence. HER-2 belongs to the human epidermal growth factor receptor family, which consists of 4 members: EGFR, HER-2, HER-3, and HER-4 (4). These receptors are located on the surface of the cell membrane and have similar structures. HER-2 expression is extremely low in normal cells, but it is expressed at high levels during embryonic development. HER-2 can spontaneously form homodimeric receptors or ligands to induce the formation of heterodimeric receptors, triggering a signal transduction network (5,6), which plays an important regulatory role in cell proliferation, differentiation, development, adhesion, and migration. The prognostic effect of HER-2 was first described about 20 years ago. Data from many studies indicate that HER-2 over-expression is linked to risk of tumor metastasis in OS patients, while some other studies have shown controversial results. In order to investigate its relationship with metastasis, we conducted a meta-analysis of all available studies on HER-2 expression and OS patients. In the current work, we confirmed that HER-2 expression is increased in metastatic OS. Therefore, our study shows that HER-2 can be used as a tool to judge the prognosis and metastasis of OS.
Methods
Search strategy and study selection
A systematic search using NCBI PubMed, the Cochrane library, Embase, ISI Web of Knowledge, Springer, CNKI, Wanfang database, Chinese VIP database and CBM was performed to investigate HER-2 expression and OS metastasis. We performed the last search on Aug 28, 2018. The following terms: (HER-2 or neu or ErbB-2 or C-erbB-2) and (osteogenic tumor or osteosarcoma) were included in the search strategy by 2 investigators (JZ and QY) independently, which was checked repeatedly and no language limitations were imposed.
Inclusion and exclusion criteria
Inclusion criteria: (I) publications were written in Chinese or English; (II) sufficient information was provided to construct the 2×2 contingency table; (III) pathological diagnosis (gold standard) was used to diagnose OS; (IV) HER-2 in OS was measured using commercial reagents.
Exclusion criteria: (I) studies absence of survival outcome were excluded; (II) studies of non-dichotomous HER-2 expression levels were excluded; (III) reviews, cell and animal experiments, case reports, correspondences, talks, expert opinions, letters, and editorials without original data were excluded; (IV) there was no cut-off value in the paper; (V) OS was diagnosed without a biopsy; (VI) multiple duplicate data were published in the different works, excluding earlier and smaller sample data; (VII) similar studies were published by the same author.
Data extraction
The eligibility of all retrieved studies was evaluated by two investigators (JZ and QY). Two investigators (JZ and QY) extracted the relevant data independently. Extracted databases were then crosschecked between the two authors to rule out any discrepancy. Data regarding the following for each included study were extracted independently: publication year, first authors’ surname, HER-2 assessment methods, and the cut-off definition. Corresponding authors were contacted if further information was needed. The study was excluded if no response was received after sending a reminder.
Assessment of included studies
We assessed the quality of included studies using NOS (7) with three categories (selection, comparability, and exposure) and eight items. The quality assessment values ranged from 0 to 9 stars. Studies with score >6 stars were included for our analysis.
Statistical analysis
The pooled odds ratio (OR) with corresponding 95% confidence interval (95% CI) was calculated to evaluate the effect of HER-2 positive expression on metastasis of OS. The heterogeneity between the included studies was assessed by I2 statistics, which quantified the proportion of the total variation in meta-analysis assessment from 0 to 100% (8). When there was no significant heterogeneity (I2≤50%), the fixed effects model was used (9); otherwise, a random effects model was used for the analysis (10). Moreover, sensitivity analysis was performed by sequentially omitting individual studies to assess the stability of the results. The possibility of publication bias was assessed via visually assessing the symmetry of Egger’s test and Begg’s funnel plots (11). All the analyses were conducted using STATA version 12 software (StataCorp LP, College Station, TX, USA). A two-tailed P<0.05 was considered statistically significant.
Results
Eligible studies
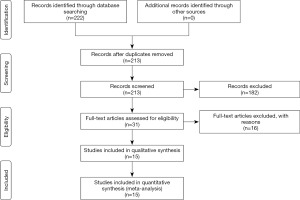
We retrieved 222 potentially relevant articles from our initial search in NCBI PubMed and so on. After removing duplicate data, 213 articles were remained. After reviewing the title and abstract, 182 manuscripts were excluded because they were either commentary or case reports, either duplicated or not relevant to the current analysis. Next, we further evaluated the remaining 31 studies. Among these, 16 studies were excluded for the following reasons: 3 items were not provided in full, 7 items were restricted to the study of molecular biology mechanisms, and 6 items did not provide enough data to calculate OR and 95% CI. A total of 652 patients participated in the current analysis in 15 studies (12-26) (Figure 1, Table 1), with 14 to 80 patients in each study (median of 47). The main features of the included studies are summarized in Table 1. In general, all studies used immunohistochemistry (IHC) to determine HER-2 expression. We judged the results by two methods of cut-off: positive cell percentage and staining intensity.
Table 1
| Ref | Study | Year | No. of patients | Age (median) | Method | Assay kit | HER-2 cut-off | HER-2 positive | HER-2 negative | NOS score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metastasis | Non metastasis | Metastasis | Non metastasis | ||||||||||
| (12) | Wang et al. | 2018 | 60 | 24 | IHC | OriGene | A1*B1>2 | 9 | 4 | 18 | 29 | 8 | |
| (13) | Qin et al. | 2017 | 45 | 28 | IHC | MXB | A2*B1≥3 | 3 | 6 | 7 | 29 | 7 | |
| (14) | Mardanpour et al. | 2016 | 28 | 26.44 | IHC | DAKO | A3≥2 | 13 | 4 | 1 | 10 | 8 | |
| (15) | Chen et al. | 2015 | 80 | 31.2 | IHC | MXB | – | 47 | 14 | 6 | 13 | 7 | |
| (16) | Becker et al. | 2013 | 27 | 13 | IHC | DAKO | A4≥2 | 1 | 2 | 7 | 17 | 7 | |
| (17) | Ma et al. | 2012 | 63 | 16 | IHC | Santa cruz | B2 | 12 | 26 | 0 | 25 | 7 | |
| (18) | Ma et al. | 2011 | 14 | 16 | IHC | Santa cruz | B2 | 3 | 0 | 5 | 6 | 8 | |
| (19) | Su et al. | 2009 | 30 | 18 | IHC | MXB | A5>1 | 3 | 15 | 2 | 10 | 7 | |
| (20) | Qiu et al. | 2006 | 69 | 17.4 | IHC | MXB | A5+B1≥4 | 19 | 9 | 10 | 31 | 8 | |
| (21) | Qiu et al. | 2006 | 38 | 17.7 | IHC | MXB | – | 10 | 5 | 6 | 17 | 7 | |
| (22) | Zhou et al. | 2003 | 25 | 14 | IHC | Ventana | A1≥2 | 7 | 4 | 5 | 9 | 8 | |
| (23) | Morris et al. | 2001 | 53 | 16.9 | IHC | DAKO | A1≥2 | 3 | 21 | 3 | 26 | 7 | |
| (24) | Kilpatrick et al. | 2001 | 41 | 29 | IHC | DAKO | B1≥2 | 14 | 26 | 0 | 1 | 7 | |
| (25) | Gorlick et al. | 1999 | 53 | 16.9 | IHC | DAKO | A2≥2 | 8 | 33 | 2 | 10 | 8 | |
| (26) | Onda et al. | 1996 | 26 | 19.9 | IHC | Nichirei Inc | – | 10 | 1 | 6 | 9 | 7 | |
A, positive cell percentage; A1, scored 1 (<25%), 2 (25–75%), 3 (>75%); A2, scored 0 (<5%), 1 (6–25%), 2 (26–50%), 3 (>50%); A3, scored 0–3+; A4, scored 0–1 (<30%), 2–3 (>30%); A5, scored 1 (>10%); B, staining intensity; B1, scored 0 (absence of staining), 1 (weak staining), 2 (moderate staining), 3 (strong staining); B2, scored by a pathologist.
Qualitative assessment
The study quality was evaluated using NOS, generating scores ranging from 7 to 8 (with a mean of 7.40). A higher value [0–9] indicates better methodology. The results of the quality assessment are shown in Table 1 and detailed information are shown in Table S1.
Meta-analysis
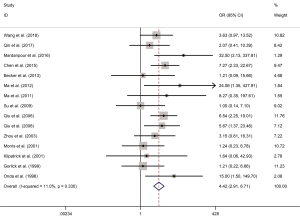
In order to evaluate the heterogeneity of the included studies (I2), a I-squared test was used, where I2 evaluated the number of inconsistencies throughout the study (P<0.05 was considered significant). The value of I2 close to zero represents homogeneity, while the following ranges of I2 are used to explain heterogeneity: low heterogeneity if I2=25–50%, moderate heterogeneity if I2=51–75% and significant heterogeneity if I2>75%. In this study, OR was used to evaluate the relationship between metastasis rate and HER-2 positive expression in patients with OS. As shown in Figure 2, the OR that assessed the HER-2 positive expression on OS metastasis was 4.42 (95% CI, 2.91–6.71; P<0.0001; I2=11.0%), which suggests a low heterogeneity among the 15 studies analyzed (I2=11.0%) in the meta-analysis of the effect of HER-2 expression on the metastasis rate of OS. Therefore, a fixed effect model was used to calculate the combined OR and the corresponding 95% CI.
Sensitivity analysis
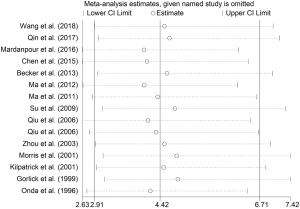
We performed a sensitivity analysis to assess the stability of the results, indicating that the combined OR was stable and there was no significant change in heterogeneity when removing a single study. We evaluated the robustness of the results by canceling one study at a time and recalculating the overall OR. A one-time sensitivity analysis was conducted to show that our analysis was not too dependent on a study and the conclusion was stable (Figure 3). These results indicate that HER-2 positive expression may be an indicator of metastasis in patients with OS.
Publication bias
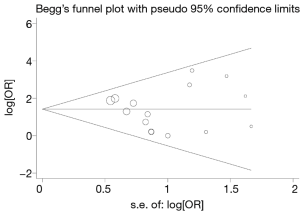
In order to test whether there is obvious evidence of asymmetry and the publication bias of the quantitative analysis literature, we used a Begg’s funnel plot with STATA. Begg’s funnel plot does not show obvious asymmetric evidence in metastasis, with a P value of 0.838 (P>0.05) (Figure 4).
Discussion
OS is the most common malignant bone tumors in adolescents and young adults. The OS prognosis is poor with low sensitivity to chemotherapy and radiotherapy. It also has the characteristics of easy recurrence and metastasis. Therapy for OS has hit the bottleneck. The 10-year survival rate of amputation therapy for primary OS patients is only 15% (2) and the combination chemotherapy does not improve OS (27). Recently, many studies about the prognosis of OS have been reported. CRP and ESR levels of pre-operation may have value in building a prognostic model for OS (28). Hypoxia-inducible factor-2 alpha (HIF2α) may be a biomarker of OS (29). Controlled and predictive marker studies of primary bone tumors was allowed by the newly developed and validated preclinical model (30). All that reports would contribute to the development of prognostic biomarker for OS. Additionally, the impact of Managed Clinical Network for Sarcoma on patient outcomes was also assessed. Previous study indicated that Scotland have similar outcomes to the UK following diagnosis of a primary malignant bone tumor (31). Although many efforts on OS have been done, the survival of patients with metastatic OS remains extremely poor. Because OS has a high degree of malignancy, early metastasis is the main factor affecting whether the patient’s cure rate can be improved. Therefore, it is urgently needed to update the early prognostic biomarkers to adapt to the appropriate treatment of malignant tumors. Many researchers report that high expression of certain cell surface markers suggests poor clinical characteristics and prognosis. HER-2 is one of the potential prognostic indicators of OS. It has been reported that the HER-2 gene is of irreplaceable significance in predicting the survival and recurrence time of many cancer patients. In terms of hormone receptor status, it has higher prognostic value than many currently used prognostic factors. HER-2 has been proved to have a role in the transfer of promoters via acting PI3K/Akt pathway to increase the proliferation of cancer cells. HER-2 gene and protein expression levels in some tumors, especially breast cancer metastases (32,33), are significantly higher than those in primary tumors. In addition, high expression of HER-2 protein was also found in the pseudopodia of invasive cancer cells, indicating that this gene is closely related to cancer metastasis. Moreover, HER-2, like other members of other tyrosine kinase receptor families, not only regulates the growth and differentiation of cells, but also influences the abilities of cells to move and adhere, and is involved in the process of metastasis of tumor cells in many ways. The high expression of HER-2 increases the proliferation, survival and anti-apoptotic capacity of tumor cells and increases the ability to migrate and infiltrate, which often indicates a poor prognosis (34). Therefore, selective HER-2 inhibitors have potential application value in the prevention of OS, enhancing the sensitivity of chemotherapy and radiotherapy, and reducing their toxic and side effects. However, whether it can be used as a new drug for the treatment of OS in clinical applications requires further research. In conclusion, HER-2 molecule is an important molecule involved in tumor growth, invasion and metastasis, and it may be a valuable prognostic biomarker in OS.
Meta-analysis is a quantitative approach combing information from different studies on related topics to facilitate the assessment of cancer-related prognostic indicators (35). In order to conduct a precise assessment about the prognostic role of HER-2 positive expression in OS, a meta-analysis was conducted and fifteen published studies was included. The results showed that HER-2 positive expression indicates higher rates of OS metastasis 4.42 (95% CI, 2.91–6.71; P<0.0001). Additionally, a sensitivity analysis was performed to determine the stability of the results. When any single study was removed, the pooled OR was stable with no significant changes. In summary, meta-analysis shows that HER-2 is a valuable biomarker to guide the clinical therapy for OS.
However, there are still several limitations in this study and following questions should be considered. Firstly, there is no publication bias in the selection of documents, but there may still be potential publication bias. Because these studies with desired results are more easily released, which may lead to bias in the overall accuracy. Secondly, the inclusion of the literature is limited to English and Chinese publications, which also have an impact on the results. Thirdly, because of the scarcity of OS, the total sample size included in this Meta-analysis was 652, of which 332 were HER-2 positive and 320 were HER-2 negative. Fewer samples will have unavoidable random errors and sample biases in the meta-analysis process. This requires us to conduct a larger sample size study to better assess the correlation between HER-2 positive expression and OS metastasis. Fourthly, tumor metastasis is used as the main outcome, but it still lacks sufficient data. We can’t stratify data according to patient age, tumor size, tumor stage, and site of metastasis. This requires a larger sample size and more detailed research program designed to more comprehensive evaluate the link of connection between the two.
In conclusion, this meta-analysis was performed to evaluate the association between HER-2 positive expression and metastasis of patients with OS. Results of this meta-analysis indicated that HER-2 was an effective biomarker that correlates with OS metastasis. More well-designed studies with larger sample sizes are still needed to obtain a more comprehensive evaluation about the prognostic role of HER-2 positive expression in OS patients.
Table S1
| Column | Entries | Study | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang et al., 2018 | Qin et al., 2017 | Mardanpour et al., 2016 | Chen et al., 2015 | Becker et al., 2013 | Ma et al., 2012 | Ma et al., 2011 | Su et al., 2009 | Qiu et al., 2006 | Qiu et al., 2006 | Zhou et al., 2003 | Morris et al., 2001 | Kilpatrick et al., 2001 | Gorlick et al., 1999 | Onda et al., 1996 | ||
| Section | Is the definition adequate | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Representativeness of the cases | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Selection of controls | ||||||||||||||||
| Definition of controls | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Comparability | Comparability of cases and controls on the basis of the design and analysis | ☆☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆☆ | ☆ | ☆☆ | ☆ | ☆ | ☆☆ | ☆ |
| Exposure | Ascertainment of exposure | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Same method of ascertainment for cases and controls | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Non-Response rate | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | |
| Total scores | 8 | 7 | 8 | 7 | 7 | 7 | 8 | 7 | 8 | 7 | 8 | 7 | 7 | 8 | 7 | |
Acknowledgments
The authors thank Dr. Ayub Abdulle nur, Dr. Chenxi Li and Dr. Zhihua Fan for English language support in preparing revised manuscript.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.26). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siclari VA, Qin L. Targeting the osteosarcoma cancer stem cell. J Orthop Surg Res 2010;5:78. [Crossref] [PubMed]
- Picci P, Mercuri M, Ferrari S, et al. Survival in high-grade osteosarcoma: improvement over 21 years at a single institution. Ann Oncol 2010;21:1366-73. [Crossref] [PubMed]
- Zhang Q, Liu F, Wang B, et al. HER-2 expression in biopsy and surgical specimen on prognosis of osteosarcoma: A systematic review and meta-analysis of 16 studies. Medicine (Baltimore) 2016;95:e3661. [Crossref] [PubMed]
- Eccles SA. The epidermal growth factor receptor/Erb-B/HER family in normal and malignant breast biology. Int J Dev Biol 2011;55:685-96. [Crossref] [PubMed]
- Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014;25:282-303. [Crossref] [PubMed]
- Roskoski R. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Comm 2004;319:1-11. [Crossref] [PubMed]
- Wells G, Shea S, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Wang SL, Zhong GX, Wang XW, et al. Prognostic significance of the expression of HER family members in primary osteosarcoma. Oncol Lett 2018;16:2185-94. [PubMed]
- Qin X, Zhang Y, Chen W, et al. Expression of EGFR and HER-2 in osteosarcoma and its clinical significance. J Clin Exp Pathol 2017;11:1275-77.
- Mardanpour K, Rahbar M, Mardanpour S. Coexistence of HER2, Ki67, and p53 in Osteosarcoma: A Strong Prognostic Factor. N Am J Med Sci 2016;8:210-4. [Crossref] [PubMed]
- Chen Z. Expression of HER-2 in osteosarcoma and screening of its adaptor method. Soochow University, 2015.
- Becker RG, Galia CR, Morini S, et al. Immunohistochemical expression of vegf and her-2 proteins in osteosarcoma biopsies. Acta Ortop Bras 2013;21:233-8. [Crossref] [PubMed]
- Ma Q, Zhou Y, Ma B, et al. The clinical value of CXCR4, HER2 and CD44 in human osteosarcoma: A pilot study. Oncol Lett 2012;3:797-801. [PubMed]
- Ma Q, Zhou Y, Jiang K, et al. Expression of HER-2 in patients with osteosarcoma. Science Technology and Engineering 2011;11:3045-8.
- Su X, Gu X, Yu G, et al. Expression of HER-2 and PTEN/mTOR in osteosarcoma and its clinical significance. Modern Oncology 2009;17:732-4.
- Qiu X, Shan L, Xu Y, et al. HER-2 gene expression and prognosis of osteosarcoma. Modern Oncology 2006;14:742-4.
- Qiu X, Shan L, Xu Y, et al. Osteosarcoma HER-2 gene expression increases the risk of lung metastasis. Journal of Practical Medicine 2006;22:123-4.
- Zhou H, Randall RL, Brothman AR, et al. Her-2/neu expression in osteosarcoma increases risk of lung metastasis and can be associated with gene amplification. J Pediatr Hematol Oncol 2003;25:27-32. [Crossref] [PubMed]
- Morris CD, Gorlick R, Huvos G, et al. Human epidermal growth factor receptor 2 as a prognostic indicator in osteogenic sarcoma. Clin Orthop Relat Res 2001;59-65. [Crossref] [PubMed]
- Kilpatrick SE, Geisinger K, King T, et al. Clinicopathologic analysis of HER-2/neu immunoexpression among various histologic subtypes and grades of osteosarcoma. Mod Pathol 2001;14:1277-83. [Crossref] [PubMed]
- Gorlick R, Huvos A, Heller G, et al. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J Clin Oncol 1999;17:2781-8. [Crossref] [PubMed]
- Onda M, Matsuda S, Higaki S, et al. ErbB-2 expression is correlated with poor prognosis for patients with osteosarcoma. Cancer 1996;77:71-8. [Crossref] [PubMed]
- Liao Z, Qiu M, Yang J, et al. Outcomes of surgery and/or combination chemotherapy for extraskeletal osteosarcoma: a single-center retrospective study from China. Sci Rep 2019;9:4816. [Crossref] [PubMed]
- Jettoo P, Tan G, Gerrand CH, et al. Role of routine blood tests for predicting clinical outcomes in osteosarcoma patients. J Orthop Surg (Hong Kong) 2019;27:2309499019838293. [Crossref] [PubMed]
- Wagner F, Holzapfel BM, Martine LC, et al. A humanized bone microenvironment un-covers HIF2 alpha as a latent marker for osteosarcoma. Acta Biomater 2019;89:372-81. [Crossref] [PubMed]
- Wagner F, Holzapfel BM, McGovern JA, et al. Humanization of bone and bone marrow in an orthotopic site reveals new potential therapeutic targets in osteosarcoma. Biomaterials 2018;171:230-46. [Crossref] [PubMed]
- MacDonald F, Gupta S. Has the Scottish Managed Clinical Network for Sarcoma influenced the survival outcomes for primary malignant bone tumours? J Orthop 2019;16:254-9. [Crossref] [PubMed]
- Ramakrishna N, Temin S, Chandarlapaty S, et al. Recommendations on Disease Management for Patients With Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36:2804-7. [Crossref] [PubMed]
- Partridge AH, Rumble RB, Carey LA, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) ad-vanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2014;32:3307-29. [Crossref] [PubMed]
- Porcelli B, Pozza A, Bizzaro N, et al. Association between stressful life events and auto-immune diseases: A systematic review and meta-analysis of retrospective case-control studies. Autoimmun Rev 2016;15:325-34. [Crossref] [PubMed]
- Xiao D, Wang Y, Xu B. Introduction and Inspiration of Meta-Analysis. Medicine & Philosophy 1998;19:179-82.