
Clinical neurological HIFU applications: the Zurich experience
Introduction
To this day, malignant, deeply seated brain tumors constitute an all but impossible challenge to curative treatment, even with state of the art multimodal therapeutic regimes, including neurosurgery, chemo- and radiotherapy. Tumor recurrence is almost unavoidable and often within months after diagnosis patients must be declared as inoperable, leaving them in a desperate condition with palliative care. Contemporary brain tumor surgery aims at substantial cytoreduction to achieve temporary tumor control, but is limited by a delicate compromise between aggressive tumor eradication and cautious preservation of patient brain functionality. Survival of brain tumor patients is therefore greatly influenced by the location, i.e., the operability of the tumor. Novel approaches to complement conventional surgery for safe tumor reduction are urgently needed and would benefit a large patient population.
Focused ultrasound has the potential to be the core technology for such an alternative. Owing to the complex physical and chemical phenomena that can be elicited by suitably parameterized acoustic fields in living tissue, a wide range of medical applications of ultrasound have emerged both for diagnostic and for therapeutic purposes. In the field of neurology particularly researchers were enthusiastic about the potential application of high intensity focused ultrasound (HIFU) for non-invasive thermal ablation of diseased brain tissue. However, it became evident early on, that transmitting ultrasound through the intact skull was a challenging task and that the margin between successful tissue ablation in the target and adverse effects on the way to target was very small (1). Ground breaking work on the way to clinical exploitation of HIFU for brain surgery was done by Lynn, Fry and colleagues in the 1950s producing reversible and irreversible lesions in the brain in preclinical animal models (2-6), and subsequently by Meyers, Fry, Heimburger and colleagues in the sixties and seventies in patients (7-9). Heimburger succeeded in treating patients suffering both from functional brain disorders and from brain tumors using an open skull approach, where he sonicated through a bone window after craniotomy. Taking advantage of the lack of ionizing radiation in HIFU treatments he even repeatedly treated tumor recurrence in some of his patients and achieved survival times in patients diagnosed with glioblastoma multiforme (GBM) of up to 13 years. However, lacking a suitable modality for reliable image guidance of these interventions the outcome of treatments was unpredictable and adverse events were difficult to control. Ultimately, Heimburger was recognized as a pioneer of the field who unfortunately was ahead of his time (9-12).
Major technological and methodological developments were needed to make non-invasive neurosurgery by transcranial focused ultrasound a clinical reality: the development of MR-compatible, large phased array transducers (13,14) to create high-energy acoustic fields with precise spatial phase control; numeric modeling of ultrasound-bone- interaction to allow for predictable transmission of acoustic energy through the intact skull bone (15,16); proton-resonance frequency shift MR thermometry (17) to monitor the acoustic heating process at the target; and system integration into clinical treatment platforms that provided both technical precision and robustness, as well as user friendly process control and workflow management.
Clinical translation of these innovations resparked interest in HIFU neurosurgery in the late 1990s when magnetic resonance imaging was proposed as a modality capable of providing closed-loop image-guidance to the HIFU intervention process, i.e., of allowing for MR-based intraoperative target delineation, local risk assessment, monitoring of lesion creation and validation of intervention endpoint. In 2006, using a commercial HIFU system designed for the treatment of uterine fibroids Ram and colleagues (11) could demonstrate the feasibility of safely ablating malignant brain tumors by MR imaging guided focused ultrasound (MRIgFUS) in three patients, although their approach still relied on a craniotomy for undisturbed acoustic access to the target volume. Around that time, the solution to this problem, namely the technical approach to focus acoustic energy through the human skull with high enough focal gain to allow for tissue ablation in the target volume without overheating the bone and adjacent brain tissue was tested by the group of Ferenc Jolesz, BWH Boston, in a cohort of three patients suffering from centrally located glioblastomas. Using a 500-element large phased array transducer, a water bolus between transducer surface and patient skull for acoustic coupling and skull cooling, and CT-based acoustic modeling of the skull bone induced phase aberration for individual correction of transducer element phase they succeeded in generating significant local heating in the tumors of all patients (18). Unfortunately, the treatments could not achieve verifiable tumor ablation and revealed the need for phased array transducers with even more elements and higher acoustic power output to conduct therapeutically effective brain treatments.
While technically successful, both the Ram and the McDannold studies revealed significant limitations of the available ultrasound technology for brain tumor ablation, namely the vulnerability of brain tumor vasculature under high mechanical stress as induced by focused ultrasound; significant geometrical restrictions to the accessible intracranial space (limited treatment envelope) imposed by the physics of acoustic waves in the applied frequency range of 650 kHz; and the inherently slow ablation rate dictated by long cooling delays between successive sonications to avoid adverse thermal effects in skull bone and adjacent tissues.
In 2008, Martin and colleagues (19) identified functional neurosurgery as a class of indications that would better harness the lesioning precision achievable with transcranial MRIgFUS (tcMRIgFUS) while avoiding the inherent difficulties of brain tumor treatments. Most stereotactic targets applied in functional neurosurgery are located in the center of the brain (within the treatment envelope) and basically represent healthy, non-pathological brain tissue, but with functional deficits. Using the newest generation of InSightec’s Neuro tcMRIgFUS treatment platform this phase I study demonstrated the feasibility and safety of tcMRgFUS for functional neurosurgery in a cohort of nine patients suffering from chronic neuropathic pain. The reason for choosing chronic pain as a pilot application was based on the fact that the central lateral nucleus of the thalamus applied in this study is relatively large (4×4×12 mm), has some margin to vulnerable neighboring neurological structures and is located in the unspecific thalamus, where lesioning is not expected to cause loss of specific neurological functions. This data was later on complemented (20), the indication extended by studies for Essential Tremor (21,22), Parkinson’s disease (23) and neuro-psychiatric disorders (24) and provided the basis for the CE marking of the ExAblate Neuro as a medical device by the end of 2012. To date, several clinical phase I and II trials investigate the feasibility and safety of tcMRIgFUS for functional neurosurgery and report encouraging results (19-21,25).
Clinical experience
In 2010, motivated by the convincing technical performance of the InSightec Neuro platform in functional neurosurgery, the researchers at the FUS Center in Zurich, Switzerland, decided to resume work on the non-invasive treatment of brain tumors. A phase I open-label, prospective clinical trial was implemented to assess the feasibility and safety of treating patients suffering from centrally located, freshly diagnosed or recurrent gliobastoma, or brain metastases of breast and lung cancers. The study protocol was approved by the involved Ethics Committees and by SwissMedic (ClinicalTrials.gov NCT01698437).
The confidence to finally success in this endeavor that had driven HIFU development for so many years was mainly based on the significant technical improvements that had been implemented in the ExAblate Neuro since the Boston study in 2006 (18). For technical performance, the system is still operating at 650 kHz but uses a more powerful 1024-element transducer to achieve the focal gain needed to reliably ablate tissue in the target volume (26). For improved image guidance, it is now integrated into 3T MR-scanners and can be complemented with dedicated MR-coils for optimized MR quality (27). For intervention safety, the patients are immobilized with a stereotactic frame, instead of a face mask and, most importantly, integrated passive cavitation detectors interrupt treatment sonications whenever acoustic signatures indicative of inertial cavitation are detected.
Technical constraints and today’s GCP standards impose strong filtering criteria on patient selection in this ongoing study. Because of the restricted intracranial volume accessible to acoustic focusing only patients being diagnosed with centrally located, ideally thalamic brain tumors, are candidates for this study. It is our experience that this selection criterion severely limits patient admission to this study. Moreover, preoperative MR screening has to confirm the technical feasibility of the planned intervention and has to exclude critical risks for intraoperative hemorrhage by identifying vascularity in and around the tumor. Previous treatments should not have created contraindications for MR imaging by inserting non-MR-safe implants, or for FUS treatment by inducing significant cysts, scar tissue or calcifications in the proximity of the target. The planned FUS intervention should not compromise the current therapeutic regime; and the targeted tumor tissue should not be too far advanced in necrotic decay and liquefaction, so as to allow for reliable MR thermometry. The patient’s condition has to be stable with a Karnofsky score above 70. As a consequence, during a 4-year screening period only three patients with a glioblastoma meeting all inclusion and exclusion criteria of the study protocol could be identified by the two neuro-oncology centers involved in this project, of which only two could be enrolled in the study with fully informed written consent.
The tcMRIgFUS intervention process has been described in detail elsewhere (28). In short, on the treatment day a stereotactic frame is fixated to the patient’s head under local anesthesia for intraoperative immobilization. The patient is positioned on the MR-table but remains fully awake and responsive during the whole intervention procedure (Figure 1).
Repeated neurological assessments before, during and after the intervention ensure stable neurological conditions and allow to detect treatment related adverse neurological symptoms. Paracetamol or ondansetron can be administered prophylactically to prevent pain or nausea, if needed. After mechanically aligning the geometric center of the transducer with the tumor volume to be ablated, the space between the transducer and the patient scalp is sealed with a flexible membrane and filled with cooled, degassed water. T2- and T1-weighted (T1W) anatomical MR images are acquired to register the FUS system coordinate space into the MR coordinate space. To clearly visualize the anatomical features of the tumor, pre-operatively acquired contrast enhanced T1 weighted MR images (T1W + C) are also registered. Furthermore, a pre-operatively acquired high resolution CT data set of the patient head is registered to the MR images for subsequent acoustic modeling and correction of skull induced acoustic distortions by the FUS system software. Thermal tissue ablation is achieved by transmitting pulses of focused ultrasound (sonications) of 10-25 seconds duration and 150-1,000 Watt acoustic power into the targeted tumor tissue where acoustic attenuation converts acoustic energy into heat. Since a substantial part of the transmitted acoustic energy is absorbed in the patient skull cooling periods of several minutes are required between sonications to prevent adverse thermal lesions in the scalp, the skull bone, or the meninges. Sonication target coordinates and sonication parameters, such as duration and acoustic power, are individually prescribed in the FUS system user interface after careful evaluation of pre- and intraoperative MR images and thermal results of previously applied sonications. For safety reasons, we decided to sonicate primarily viable centrally located tumor tissue that does not involve functionally significant eloquent structures, although we are aware of the fact that for complete tumor eradication the peripheral area beyond the gadolinium enhancing tumor volume, i.e., the penumbra, where cancer cells are invading healthy brain tissue should be included into the treatment volume. The total duration of tcMRIgFUS interventions generally lasts between 4 and 5 h. In this study, follow-up assessment includes neurological examinations and MR imaging immediately following the operation, and at 2, 30 and 90 days thereafter.
Treatment 1
Patient 1 (32 years old, male) was referred to the neurosurgical department because of a cystic mass in the left sided thalamus extending into the ipsilateral cerebral peduncle. Neuroradiological workup and stereotactic biopsy classified the tumor as a grade IV, wild type GBM. Due to central location and infiltration of the left thalamus the tumor was considered to be inaccessible for resection by classical neurosurgery and the patient received chemo- and radiotherapy. Despite therapeutic efforts the tumor continued to grow rapidly and subsequently blocked the circulation of the CSF requiring a ventriculo-peritoneal (vp) shunt. In April 2012, when the tumor size/volume reached 5 cm × 7 cm/75 cc, respectively, the tumor board proposed the patient as a potential candidate for participation in our tcMRIgFUS phase I tumor study.
Planning of the tcMRIgFUS treatment was complicated by a vp shunt whose tip was situated close to the target volume and its drainage line on the scalp that had to be excluded from the sonication pathway (Figure 2).

The treatment started successfully according to protocol but stalled during focal spot localization. Although up to 450 W acoustic power were applied during sonications 1 through 8, a power setting that is supposed to induce focal heating up to 54 °C according to our previous treatment experience, MR thermometry failed to measure correct thermal rise. Therefore, lacking reliable temperature monitoring the treatment was prematurely terminated for safety reasons.
Post intervention analysis of CT, MR imaging and MR thermometry data led to the conclusion that the tip of the shunt contained a small ferromagnetic contamination. The local B0 inhomogeneity induced by this particle caused significant off-resonance effects in the MR signal that resulted in unreliable thermal measurements. Nevertheless, while the patient’s chemotherapeutic regimens were kept unchanged as required by his treatment protocol, no further tumor progression could be detected during the following 10 months. The patient’s overall clinical presentation improved and allowed him to leave the hospital, return to his family and even to resume professional work part time again.
Treatment 2
After a 10-month period of well-being of patient 1, the tumor began to regrow again. A second tcMRIgFUS intervention was attempted in February 2013. Sadly, on the day of the intervention the clinical condition of the patient had deteriorated so much that he could not tolerate the motionless supine position required for the procedure for an extended period of time. Even though a peak temperature of 47 °C could be achieved the procedure had to be aborted prematurely, again without resulting in tumor ablation.
Treatment 3
Patient 2 (63 years old, male) presented with a rapidly growing recurrent tumor in the left thalamic and subthalamic region 5 years after first surgical resection of a GBM in the temporal lobe. The location of the recurrent tumor in eloquent brain areas precluded surgical reevaluation. The patient’s neurological symptoms included a right-sided facio-brachio-crural hemiparesis and a slight esophoria with ptosis of the right eye. In a preoperative MR-angiogram pronounced vascularization of the tumor regions could be excluded, and thus, the tumor board proposed a tcMRIgFUS intervention.
The treatment was conducted in March 2014 (29) according to the study protocol. A total of 25 sonications were applied with up to 19,550 Joules of acoustic energy, of which 17 reached ablative temperatures of over 55 °C with a maximum of 65 °C, as recorded by intra-operative real-time MR thermometry (Figure 3).

According to the purpose of the clinical study, the treatment was ended when intra-operative real-time MR thermometry and calculated thermal dose maps predicted successful ablation of a substantial volume of the tumor tissue, thereby having established the clinical feasibility of the procedure.
MR images acquired immediately after the intervention revealed multiple isolated hyperintense lesions on diffusion tensor MR images (DTI) in the sonicated tumor part. No signs indicative of intracranial hemorrhage or perifocal edema at the sites of presumably ablated tissue were visible. On day 5 comparison of pre- and post-operative, contrast enhanced T1-weighted MRI confirmed initial DTI findings and revealed new, well defined areas of non-enhancing, and thus non perfused, coagulated tumor tissue showing high DTI signals as expected (Figure 4).
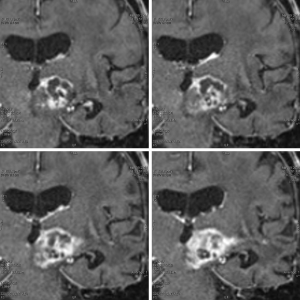
The cumulative ablated tumor volume calculated by manual delineation on T1W+C MRI was 0.7 cc corresponding to 10% of the enhancing tumor volume of 6.5 cc. Neurological examination on day 5 post-op revealed an improvement of the patient’s general condition compared the preoperative state with a substantial amelioration of the hemiparesis of his right arm and a resolution of the ptosis of his right eyelid. No treatment related neurological adverse effects were observed. Repeated follow-up examinations until the time of writing this report, 6 months after the HIFU intervention, find unchanged patterns of ablated tumor tissue and no signs of tumor progression. The patient enjoys a reasonably good health condition and is partially ambulant at home together with his family.
Discussion
Both, the failures in treatments 1 and 2, and the success in treatment 3 teach us important lessons on the clinical potential of tcMRIgFUS for brain tumor ablation.
In the first two interventions the treatment team was confronted with the full spectrum of comorbidities typically associated with advanced brain tumor progression and which, notably, are mostly avoided by functional tcMRIgFUS neurosurgery. Brain tumor patients in advanced stage are often in a precarious clinical state while the tcMRIgFUS intervention requires the patients to stay immobilized in supine position in the MR bore for several hours keeping awake for neurological monitoring. They carry artifacts of previous and current treatments such as scars, calcifications, bone replacements, subcutaneous device for drug delivery, shunts, clips and other implants while the acoustic path to the target volume should be free of absorbing or scattering materials and no MRI-unsafe or MRI distorting implants should be present. The limitations imposed by these conditions might be alleviated through sophisticated anesthesiological management and adapted treatment and imaging protocols, but ultimately they will be hard to overcome.
Accordingly, the success in treatment 3 can be attributed largely to the fact that the patient presented in a reasonably good clinical condition and was referred to tcMRIgFUS with a freshly diagnosed, but rapidly growing recurrent tumor after years of chemotherapy only, thus offering an operation field that was, acoustically speaking, not compromised by earlier treatments. Still, even under these optimal conditions the total ablation volume achieved in the 4 h treatment session was only 0.7 cc, i.e., only 10% of the enhancing tumor volume, and not nearly sufficient for significant cytoreduction as is believed to be the key for sustained tumor control. Even though the reduction of space-occupying and displacing effects of the tumor mass resulted in immediate improvement of neurological condition and quality of life for the patient, the unfavorable relationship between achievable ablation volume per sonication and total tumor volume suggests the application of tcMRIgFUS mostly for local tumor control.
Nevertheless, treatment 3 also confirmed the expected benefits of tcMRIgFUS as a non-invasive, fully image guided and controlled thermal intervention modality. Precise intraoperative MR image guidance allowed to selectively target viable gadolinium enhancing tumor tissue and to operate in close vicinity of the tumor border. Eloquent neurological structures could be spared and collateral damage avoided by integrating anatomical information provided by preoperative high-resolution MRI. Although thermal lesions are induced immediately through deposited acoustic energy local anatomy is not distorted by the lesions and therefore, preoperative structural or physiological data registered into the FUS coordinate system remains valid throughout the procedure. In contrast to classical surgical interventions, repeated examinations of the fully awake and communicating patient allowed to assure the integrity of his neurological status during the entire tcMRIgFUS intervention and immediately thereafter. Treatment progress could be monitored by intraoperative MR thermometry and thermal dose maps predicted treatment outcome. Owing to the non-invasive approach of tcMRIgFUS the patient could leave the hospital on his own wish on day 2 after the intervention in an even better condition than before. This is all the more remarkable as the intervention had thermally deactivated significant portions of tumor tissue. The risk of allowing the patient to leave the hospital so early was mitigated by careful post-operative neuroradiological workup that allowed to unambiguously evaluate the thermal lesions which, in contrary to radiation necrosis, appear immediately after the sonications and are known to be stable in size, or even shrink over time. Finally, since tcMRIgFUS does not involve ionizing radiation the treatment 3 can be repeated if needed, i.e., the patient is, in accordance with the clinical protocol, eligible for further global tumor mass reduction or local control of resumed tumor growth by tcMRIgFUS if considered indicated by the tumor board.
The clinical status of the second patient after treatment 3 up to the time of writing this report is remarkable. Together with his improved neurological condition a complete stop of volume increase or progression of the previously rapidly growing tumor is observed. Lacking histological or biochemical evidence for local physiological reactions, one can only speculate about the reasons for this observation and its duration. Potential explanations are a local sensitization of tumor cells to the chemotherapy in proximity to ablation sites by physiological effects similar to hyperthermia; a temporary increase in the local concentration and thus the efficacy of the currently used chemotherapeutic agent owing to transient heat induced opening of the blood brain and blood tumor barriers around the sonication sites; or a systemic anti-tumor response triggered by presenting tumor antigens from thermally damaged tumor cells to the immune system as is reported from thermal ablation of tumors (30). We consider this to be an important clinical observation that relates to earlier reports from Heimburger (9) and Ram (11) describing prolonged survival of patients with malignant gliomas after HIFU interventions. Hyperthermia is known to regulate various molecular aspects of the immune response (31,32). Therefore, HIFU induced whole or even partial tumor cell necrosis by heat coagulation or hyperthermia, respectively, may well induce different anti-tumor responses by the innate and adaptive immune system. Tentative evidence for such HIFU-augmented specific host anti-tumor immune response can be found in experimental (33-38) and clinical reports (9,11,39). In addition, similar observations are reported for extracranial, solid tumors (40-42), including regression or even complete resolution of distant untreated tumors and metastases after partial thermal ablation or cryo-therapy [for review see (43)].
Conclusions
The preliminary technical and clinical results of the three tcMRIgFUS treatments conducted so far in this ongoing study demonstrate that noninvasive focused ultrasound interventions for brain tumors can be safely carried out and can create clinical benefits for the patient. They also illustrate how targeting and lesioning precision exploited so far only in functional tcMRIgFUS neurosurgery creates a clinical benefit in tumor treatments too: specific areas of the tumor and in particular the tumor border can be ablated accurately whereas supposedly eloquent areas in the penumbra of the tumor can be spared. Also, while tcMRIgFUS suffers from severe restrictions in the accessible brain volume in the periphery of the brain, e.g., in the cerebral cortex, it can safely access deep seated brain tumors not amendable for conventional neurosurgery without inflicting collateral damage on the way to the target. These encouraging results reinforce the huge expectations towards HIFU as a new core technology for neurological applications. However, both the failures in treatments 1 and 2 under very difficult conditions, and the success in treatment 3 under optimal conditions show that there is still a wide gap to widespread clinical application. Substantial technical challenges, such as widening the accessible brain area or increasing the achievable ablation volume per treatment time remain to be overcome in order to establish tcMRIgFUS as a prevailing neurosurgical modality for brain tumor treatment. Technical evolution will show whether it will become a versatile tool for bulk tumor ablation or a specialist tool for ablation of residual tumor foci after classical resection.
While context and available space have limited this account to clinical experience with HIFU for thermal ablation of brain tumors, clinical translation of pulsed low intensity ultrasound and systemically administered microbubbles for transient opening of the blood brain barrier for targeted drug delivery into the brain parenchyma is imminent and has the potential to constitute radically new treatment paradigms (44,45). Such new paradigms will ultimately be based on variations of the tcMRIgFUS technology described here. Exploiting not only the thermal effects of ultrasound but the whole spectrum of physical and physiological reactions observed in the interaction of ultrasound with living cells to treat brain tumors will be again a long endeavor requiring interdisciplinary research through a wide range of natural science, medicine and engineering disciplines.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giusi Irma Forte and Giorgio Russo) for the series “High intensity focused ultrasounds” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.09.01). The series “High intensity focused ultrasounds” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lynn JG, Zwemer RL, Chick AJ. The biological application of focused ultrasonic waves. Science 1942;96:119-20. [PubMed]
- Lynn JG, Putnam TJ. Histology of Cerebral Lesions Produced by Focused Ultrasound. Am J Pathol 1944;20:637-49. [PubMed]
- Fry WJ. Intense ultrasound; a new tool for neurological research. J Ment Sci 1954;100:85-96. [PubMed]
- Fry WJ, Mosberg WH Jr, Barnard JW, et al. Production of focal destructive lesions in the central nervous system with ultrasound. J Neurosurg 1954;11:471-8. [PubMed]
- Fry WJ, Barnard JW, Fry EJ, et al. Ultrasonic lesions in the mammalian central nervous system. Science 1955;122:517-8. [PubMed]
- Fry FJ, Ades HW, Fry WJ. Production of reversible changes in the central nervous system by ultrasound. Science 1958;127:83-4. [PubMed]
- Meyers R, Fry WJ, Fry FJ, et al. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J Neurosurg 1959;16:32-54. [PubMed]
- Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron 1960;ME-7:166-81. [PubMed]
- Heimburger RF. Ultrasound augmentation of central nervous system tumor therapy. Indiana Med 1985;78:469-76. [PubMed]
- Park JW, Jung S, Junt TY, et al. Focused ultrasound surgery for the treatment of recurrent anaplastic astrocytoma: A preliminary report. AIP Conf Proc 2006;829:238-40.
- Ram Z, Cohen ZR, Harnof S, et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery 2006;59:949-55; discussion 955-6. [PubMed]
- Fry FJ, Johnson LK. Tumor irradiation with intense ultrasound. Ultrasound Med Biol 1978;4:337-41. [PubMed]
- Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol 1998;24:275-83. [PubMed]
- Hynynen K, Clement GT, McDannold N, et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: a preliminary rabbit study with ex vivo human skulls. Magn Reson Med 2004;52:100-7. [PubMed]
- Tanter M, Thomas JL, Fink M. Focusing and steering through absorbing and aberrating layers: application to ultrasonic propagation through the skull. J Acoust Soc Am 1998;103:2403-10. [PubMed]
- Clement GT, Hynynen K. A non-invasive method for focusing ultrasound through the human skull. Phys Med Biol 2002;47:1219-36. [PubMed]
- Ishihara Y, Calderon A, Watanabe H, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med 1995;34:814-23. [PubMed]
- McDannold N, Clement GT, Black P, et al. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery 2010;66:323-32; discussion 332. [PubMed]
- Martin E, Jeanmonod D, Morel A, et al. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol 2009;66:858-61. [PubMed]
- Jeanmonod D, Werner B, Morel A, et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus 2012;32:E1 [PubMed]
- Elias WJ, Huss D, Voss T, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2013;369:640-8. [PubMed]
- Chang WS, Jung HH, Kweon EJ, et al. Unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor: practices and clinicoradiological outcomes. J Neurol Neurosurg Psychiatry 2014; [Epub ahead of print]. [PubMed]
- Bauer R, Martin E, Haegele-Link S, et al. Noninvasive functional neurosurgery using transcranial MR imaging-guided focused ultrasound. Parkinsonism Relat Disord 2014;20:S197-9. [PubMed]
- Chang WS, Roh D, Kim CH, et al. Combined bilateral anterior cingulotomy and ventral capsule/ventral striatum deep brain stimulation for refractory obsessive-compulsive disorder with major depression: do combined procedures have a long-term benefit? Restor Neurol Neurosci 2013;31:723-32. [PubMed]
- Lipsman N, Schwartz ML, Huang Y, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol 2013;12:462-8. [PubMed]
- McDannold N, Werner B, Jeanmonod D, et al. MRI-based temperature analysis of transcranial MRI-guided focused ultrasound surgery for functional neurosurgery. Proc Intl Soc Magn Reson Med 2010;18:4122.
- Werner B, Martin E, Bauer R, et al. Optimizing MR imaging-guided navigation for focused ultrasound interventions in the brain. ISTU Proceedings 2014. (In press).
- Martin E, Werner B. Focused Ultrasound of the Brain. Curr Radiol Rep 2013;1:126-35.
- Coluccia D, Fandino J, Schwyzer L, et al. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. Therapeutic Ultrasound 2014; In press.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014;14:199-208. [PubMed]
- Zheng H, Benjamin IJ, Basu S, et al. Heat shock factor 1-independent activation of dendritic cells by heat shock: implication for the uncoupling of heat-mediated immunoregulation from the heat shock response. Eur J Immunol 2003;33:1754-62. [PubMed]
- Cippitelli M, Fionda C, Di Bona D, et al. Hyperthermia enhances CD95-ligand gene expression in T lymphocytes. J Immunol 2005;174:223-32. [PubMed]
- Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 2012;28:528-42. [PubMed]
- Calderwood SK, Theriault J, Gray PJ, et al. Cell surface receptors for molecular chaperones. Methods 2007;43:199-206. [PubMed]
- Burks SR, Ziadloo A, Hancock HA, et al. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS One 2011;6:e24730 [PubMed]
- Haen SP, Gouttefangeas C, Schmidt D, et al. Elevated serum levels of heat shock protein 70 can be detected after radiofrequency ablation. Cell Stress Chaperones 2011;16:495-504. [PubMed]
- Xia JZ, Xie FL, Ran LF, et al. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med Biol 2012;38:1363-71. [PubMed]
- Alkins R, Burgess A, Ganguly M, et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res 2013;73:1892-9. [PubMed]
- Zhou Q, Zhu XQ, Zhang J, et al. Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment. Ultrasound Med Biol 2008;34:81-7. [PubMed]
- Sánchez-Ortiz RF, Tannir N, Ahrar K, et al. Spontaneous regression of pulmonary metastases from renal cell carcinoma after radio frequency ablation of primary tumor: an in situ tumor vaccine? J Urol 2003;170:178-9. [PubMed]
- Shmilovici A. Incomplete tumor volume reduction may improve cancer prognosis. Med Hypotheses 2007;68:1236-9. [PubMed]
- Sung HY, Cho SH, Kim JI, et al. High intensity focused ultrasound therapy resulted in a complete response in a patient with advanced gastric cancer with liver metastases: a case report. Eur J Gastroenterol Hepatol 2008;20:707-9. [PubMed]
- Hu Z, Yang XY, Liu Y, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med 2007;5:34. [PubMed]
- Kovacs Z, Werner B, Rassi A, et al. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J Control Release 2014;187:74-82. [PubMed]
- McDannold N, Arvanitis CD, Vykhodtseva N, et al. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res 2012;72:3652-63. [PubMed]


